A systematic review of the effectiveness of adalimumab
A systematic review of the effectiveness of adalimumab
A systematic review of the effectiveness of adalimumab
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
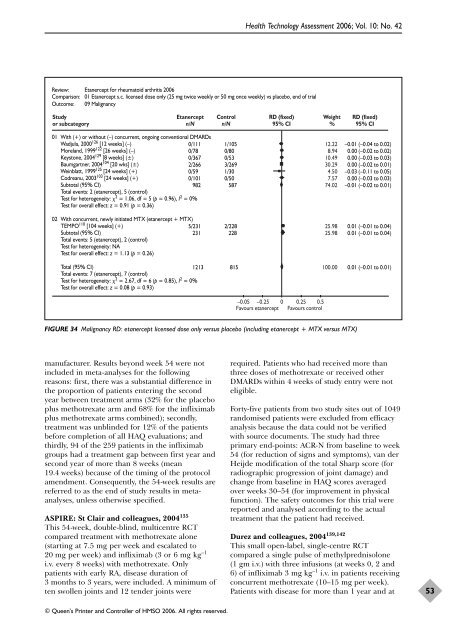
Review: Etanercept for rheumatoid arthritis 2006<br />
Comparison: 01 Etanercept s.c. licensed dose only (25 mg twice weekly or 50 mg once weekly) vs placebo, end <strong>of</strong> trial<br />
Outcome: 09 Malignancy<br />
Study<br />
or subcategory<br />
Etanercept<br />
n/N<br />
manufacturer. Results beyond week 54 were not<br />
included in meta-analyses for <strong>the</strong> following<br />
reasons: first, <strong>the</strong>re was a substantial difference in<br />
<strong>the</strong> proportion <strong>of</strong> patients entering <strong>the</strong> second<br />
year between treatment arms (32% for <strong>the</strong> placebo<br />
plus methotrexate arm and 68% for <strong>the</strong> infliximab<br />
plus methotrexate arms combined); secondly,<br />
treatment was unblinded for 12% <strong>of</strong> <strong>the</strong> patients<br />
before completion <strong>of</strong> all HAQ evaluations; and<br />
thirdly, 94 <strong>of</strong> <strong>the</strong> 259 patients in <strong>the</strong> infliximab<br />
groups had a treatment gap between first year and<br />
second year <strong>of</strong> more than 8 weeks (mean<br />
19.4 weeks) because <strong>of</strong> <strong>the</strong> timing <strong>of</strong> <strong>the</strong> protocol<br />
amendment. Consequently, <strong>the</strong> 54-week results are<br />
referred to as <strong>the</strong> end <strong>of</strong> study results in metaanalyses,<br />
unless o<strong>the</strong>rwise specified.<br />
ASPIRE: St Clair and colleagues, 2004 135<br />
This 54-week, double-blind, multicentre RCT<br />
compared treatment with methotrexate alone<br />
(starting at 7.5 mg per week and escalated to<br />
20 mg per week) and infliximab (3 or 6 mg kg –1<br />
i.v. every 8 weeks) with methotrexate. Only<br />
patients with early RA, disease duration <strong>of</strong><br />
3 months to 3 years, were included. A minimum <strong>of</strong><br />
ten swollen joints and 12 tender joints were<br />
Control<br />
n/N<br />
01 With (+) or without (–) concurrent, ongoing conventional DMARDs<br />
Wadjula, 2000126 [12 weeks] (–)<br />
Moreland, 1999122 [26 weeks] (–)<br />
Keystone, 2004129 [8 weeks] (±)<br />
Baumgartner, 2004104 [20 wks] (±)<br />
Weinblatt, 1999125 [24 weeks] (+)<br />
Codreanu, 2003103 [24 weeks] (+)<br />
Subtotal (95% CI)<br />
Total events: 2 (etanercept), 5 (control)<br />
Test for heterogeneity: 2 = 1.06, df = 5 (p = 0.96), I2 0/111<br />
0/78<br />
0/367<br />
2/266<br />
0/59<br />
0/101<br />
1/105<br />
0/80<br />
0/53<br />
3/269<br />
1/30<br />
0/50<br />
982 587<br />
= 0%<br />
Test for overall effect: z = 0.91 (p = 0.36)<br />
02 With concurrent, newly initiated MTX (etanercept + MTX)<br />
TEMPO 110 [104 weeks] (+)<br />
Subtotal (95% CI)<br />
Total events: 5 (etanercept), 2 (control)<br />
Test for heterogeneity: NA<br />
Test for overall effect: z = 1.13 (p = 0.26)<br />
5/231 2/228<br />
231 228<br />
Total (95% CI)<br />
Total events: 7 (etanercept), 7 (control)<br />
Test for heterogeneity: 2 = 2.67, df = 6 (p = 0.85), I2 1213 815<br />
= 0%<br />
Test for overall effect: z = 0.08 (p = 0.93)<br />
© Queen’s Printer and Controller <strong>of</strong> HMSO 2006. All rights reserved.<br />
Health Technology Assessment 2006; Vol. 10: No. 42<br />
RD (fixed)<br />
95% CI<br />
–0.05 –0.25 0 0.25 0.5<br />
Favours etanercept Favours control<br />
Weight<br />
%<br />
FIGURE 34 Malignancy RD: etanercept licensed dose only versus placebo (including etanercept + MTX versus MTX)<br />
12.22<br />
8.94<br />
10.49<br />
30.29<br />
4.50<br />
7.57<br />
74.02<br />
25.98<br />
25.98<br />
100.00<br />
RD (fixed)<br />
95% CI<br />
–0.01 (–0.04 to 0.02)<br />
0.00 (–0.02 to 0.02)<br />
0.00 (–0.03 to 0.03)<br />
0.00 (–0.02 to 0.01)<br />
–0.03 (–0.11 to 0.05)<br />
0.00 (–0.03 to 0.03)<br />
–0.01 (–0.02 to 0.01)<br />
0.01 (–0.01 to 0.04)<br />
0.01 (–0.01 to 0.04)<br />
0.01 (–0.01 to 0.01)<br />
required. Patients who had received more than<br />
three doses <strong>of</strong> methotrexate or received o<strong>the</strong>r<br />
DMARDs within 4 weeks <strong>of</strong> study entry were not<br />
eligible.<br />
Forty-five patients from two study sites out <strong>of</strong> 1049<br />
randomised patients were excluded from efficacy<br />
analysis because <strong>the</strong> data could not be verified<br />
with source documents. The study had three<br />
primary end-points: ACR-N from baseline to week<br />
54 (for reduction <strong>of</strong> signs and symptoms), van der<br />
Heijde modification <strong>of</strong> <strong>the</strong> total Sharp score (for<br />
radiographic progression <strong>of</strong> joint damage) and<br />
change from baseline in HAQ scores averaged<br />
over weeks 30–54 (for improvement in physical<br />
function). The safety outcomes for this trial were<br />
reported and analysed according to <strong>the</strong> actual<br />
treatment that <strong>the</strong> patient had received.<br />
Durez and colleagues, 2004 139,142<br />
This small open-label, single-centre RCT<br />
compared a single pulse <strong>of</strong> methylprednisolone<br />
(1 gm i.v.) with three infusions (at weeks 0, 2 and<br />
6) <strong>of</strong> infliximab 3 mg kg –1 i.v. in patients receiving<br />
concurrent methotrexate (10–15 mg per week).<br />
Patients with disease for more than 1 year and at<br />
53