A systematic review of the effectiveness of adalimumab
A systematic review of the effectiveness of adalimumab A systematic review of the effectiveness of adalimumab
150 Appendix 4 TABLE 73 Meta-analyses: etanercept s.c. all doses (including sublicence doses) versus placebo (with or without ongoing conventional DMARDs), end of trial Comparison or outcome Studies N included Statistical Effect size (95% CI) in analysis method ACR20 responder 7103,121,122,125,126,129,130 1672 RR (fixed) 3.48 (2.78 to 4.35)* ACR50 responder 7103,121,122,125,126,129,130 1672 RR (fixed) 4.97 (3.40 to 7.27)* ACR70 responder 6103,122,125,126,129,130 1492 RR (fixed) 8.55 (3.59 to 20.37)* RD ACR20 responder 7103,121,122,125,126,129,130 1672 RD (fixed) 0.43 (0.38 to 0.47)* RD ACR50 responder 7103,121,122,125,126,129,130 1672 RD (fixed) 0.26 (0.22 to 0.30)* RD ACR70 responder 6103,122,125,126,129,130 1492 RD (fixed) 0.11 (0.08 to 0.14)* SJC, end of study result 7103,121,122,125,126,129,130 1689 WMD (random) –5.78 (–8.12 to –3.43)* Patient’s global assessment, end of study 7 103,121,122,125,126,129,130 1689 WMD –2.33 (–2.56 to –2.10)* result (fixed) HAQ, end of study result 6 103,122,125,126,129,130 1440 WMD (fixed) –0.49 (–0.57 to –0.40)* DAS, end of study result 1 103 150 WMD (fixed) –1.50 (–1.89 to –1.11)* Modified van de Heijde–Sharp score, 0 0 Not No data available mean change from baseline estimable Withdrawal for any reasons 7 103,104,121,122,125,126,129 2168 RR (fixed) 0.43 (0.36 to 0.51)* Withdrawal due to lack of efficacy 6103,104,121,122,125,126 1748 RR (fixed) 0.28 (0.21 to 0.36)* Withdrawal due to adverse events 7103,104,121,122,125,126,129 2168 RR (fixed) 0.87 (0.54 to 1.38) Death 7103,104,121,122,125,126,129 2168 RR (fixed) 1.44 (0.44 to 4.69) SAEs 5103,104,122,125,129 1429 RR (fixed) 1.25 (0.76 to 2.06) Malignancy: all 6103,104,122,125,126,129 1988 RR (fixed) 0.47 (0.13 to 1.67) Malignancy: skin cancer excluding 6103,104,122,125,126,129 melanoma 1988 RR (fixed) 0.64 (0.15 to 2.77) Malignancy: all cancer excluding 6103,104,122,125,126,129 non-melanoma skin cancer 1988 RR (fixed) 0.34 (0.07 to 1.74) Serious infection 7103,104,122,125,126,129,130 2046 RR (fixed) 0.75 (0.37 to 1.48) Any infection 6103,104,122,125,126,129 1988 RR (random) 1.01 (0.83 to 1.24) * Statistically significant result (p < 0.05).
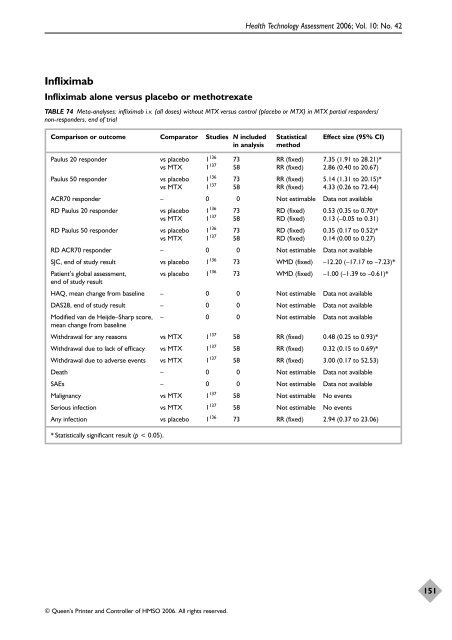
Infliximab Infliximab alone versus placebo or methotrexate © Queen’s Printer and Controller of HMSO 2006. All rights reserved. Health Technology Assessment 2006; Vol. 10: No. 42 TABLE 74 Meta-analyses: infliximab i.v. (all doses) without MTX versus control (placebo or MTX) in MTX partial responders/ non-responders, end of trial Comparison or outcome Comparator Studies N included Statistical Effect size (95% CI) in analysis method Paulus 20 responder vs placebo 1136 73 RR (fixed) 7.35 (1.91 to 28.21)* vs MTX 1 137 58 RR (fixed) 2.86 (0.40 to 20.67) Paulus 50 responder vs placebo 1136 73 RR (fixed) 5.14 (1.31 to 20.15)* vs MTX 1 137 58 RR (fixed) 4.33 (0.26 to 72.44) ACR70 responder – 0 0 Not estimable Data not available RD Paulus 20 responder vs placebo 1136 73 RD (fixed) 0.53 (0.35 to 0.70)* vs MTX 1 137 58 RD (fixed) 0.13 (–0.05 to 0.31) RD Paulus 50 responder vs placebo 1136 73 RD (fixed) 0.35 (0.17 to 0.52)* vs MTX 1 137 58 RD (fixed) 0.14 (0.00 to 0.27) RD ACR70 responder – 0 0 Not estimable Data not available SJC, end of study result vs placebo 1136 73 WMD (fixed) –12.20 (–17.17 to –7.23)* Patient’s global assessment, vs placebo 1136 end of study result 73 WMD (fixed) –1.00 (–1.39 to –0.61)* HAQ, mean change from baseline – 0 0 Not estimable Data not available DAS28, end of study result – 0 0 Not estimable Data not available Modified van de Heijde–Sharp score, mean change from baseline – 0 0 Not estimable Data not available Withdrawal for any reasons vs MTX 1137 58 RR (fixed) 0.48 (0.25 to 0.93)* Withdrawal due to lack of efficacy vs MTX 1137 58 RR (fixed) 0.32 (0.15 to 0.69)* Withdrawal due to adverse events vs MTX 1137 58 RR (fixed) 3.00 (0.17 to 52.53) Death – 0 0 Not estimable Data not available SAEs – 0 0 Not estimable Data not available Malignancy vs MTX 1137 58 Not estimable No events Serious infection vs MTX 1137 58 Not estimable No events Any infection vs placebo 1136 73 RR (fixed) 2.94 (0.37 to 23.06) * Statistically significant result (p < 0.05). 151
- Page 115 and 116: TABLE 46 Unit costs for tests and v
- Page 117 and 118: following properties, according to
- Page 119 and 120: TABLE 52 Base case: TNF inhibitors
- Page 121 and 122: TABLE 54 Base case: TNF inhibitors
- Page 123 and 124: TABLE 59 Third TNF inhibitor follow
- Page 125 and 126: TABLE 65 Sensitivity analyses: TNF
- Page 127 and 128: TABLE 67 Sensitivity analyses: TNF
- Page 129: The substantial economic impact of
- Page 133 and 134: Summary Effectiveness: principal fi
- Page 135 and 136: inhibitors, although the incrementa
- Page 137 and 138: introduce bias which generally exag
- Page 139: Adalimumab, etanercept and inflixim
- Page 143 and 144: 1. Jobanputra P, Barton P, Bryan S,
- Page 145 and 146: 46. Young A, Dixey J, Cox N, Davies
- Page 147 and 148: tumor necrosis factor therapy in th
- Page 149 and 150: arthritis: a 12-week, double-blind,
- Page 151 and 152: 171. Geborek P, Crnkic M, Petersson
- Page 153 and 154: 216. Schotte H, Willeke P, Mickholz
- Page 155 and 156: The Health Assessment Questionnaire
- Page 157 and 158: Cochrane Library (CENTRAL) 2005 Iss
- Page 159 and 160: Appendix 3 © Queen’s Printer and
- Page 161: TABLE 69 Studies excluded from clin
- Page 164 and 165: 148 Appendix 4 TABLE 71 Meta-analys
- Page 168 and 169: 152 Appendix 4 Infliximab versus pl
- Page 170 and 171: 154 Appendix 4 Infliximab plus MTX
- Page 173: Ovid MEDLINE(R) 1966 to February we
- Page 177 and 178: Appendix 8 © Queen’s Printer and
- Page 179 and 180: TABLE 79 Wong et al., 2002 161 © Q
- Page 181 and 182: TABLE 80 Kobelt et al., 2003 162 (c
- Page 183 and 184: TABLE 82 Brennan et al., 2004 160
- Page 185 and 186: TABLE 83 Kobelt et al., 2004 163 (c
- Page 187 and 188: TABLE 85 Bansback et al., 2005 166
- Page 189: TABLE 86 Kobelt et al., 2005 167 (c
- Page 192 and 193: 176 Appendix 9 TABLE 89 Strategy se
- Page 195 and 196: Extensive sensitivity analysis was
- Page 197 and 198: TABLE 96 Variation 1: TNF inhibitor
- Page 199 and 200: TABLE 99 Variation 2: TNF inhibitor
- Page 201 and 202: TABLE 102 Variation 3: TNF inhibito
- Page 203 and 204: TABLE 105 Variation 3: TNF inhibito
- Page 205 and 206: TABLE 108 Variation 4: TNF inhibito
- Page 207 and 208: TABLE 111 Variation 5: TNF inhibito
- Page 209 and 210: TABLE 114 Variation 6: TNF inhibito
- Page 211 and 212: TABLE 117 Variation 6: TNF inhibito
- Page 213 and 214: TABLE 120 Variation 7: TNF inhibito
- Page 215 and 216: TABLE 123 Variation 8: TNF inhibito
Infliximab<br />
Infliximab alone versus placebo or methotrexate<br />
© Queen’s Printer and Controller <strong>of</strong> HMSO 2006. All rights reserved.<br />
Health Technology Assessment 2006; Vol. 10: No. 42<br />
TABLE 74 Meta-analyses: infliximab i.v. (all doses) without MTX versus control (placebo or MTX) in MTX partial responders/<br />
non-responders, end <strong>of</strong> trial<br />
Comparison or outcome Comparator Studies N included Statistical Effect size (95% CI)<br />
in analysis method<br />
Paulus 20 responder vs placebo 1136 73 RR (fixed) 7.35 (1.91 to 28.21)*<br />
vs MTX 1 137 58 RR (fixed) 2.86 (0.40 to 20.67)<br />
Paulus 50 responder vs placebo 1136 73 RR (fixed) 5.14 (1.31 to 20.15)*<br />
vs MTX 1 137 58 RR (fixed) 4.33 (0.26 to 72.44)<br />
ACR70 responder – 0 0 Not estimable Data not available<br />
RD Paulus 20 responder vs placebo 1136 73 RD (fixed) 0.53 (0.35 to 0.70)*<br />
vs MTX 1 137 58 RD (fixed) 0.13 (–0.05 to 0.31)<br />
RD Paulus 50 responder vs placebo 1136 73 RD (fixed) 0.35 (0.17 to 0.52)*<br />
vs MTX 1 137 58 RD (fixed) 0.14 (0.00 to 0.27)<br />
RD ACR70 responder – 0 0 Not estimable Data not available<br />
SJC, end <strong>of</strong> study result vs placebo 1136 73 WMD (fixed) –12.20 (–17.17 to –7.23)*<br />
Patient’s global assessment, vs placebo 1136 end <strong>of</strong> study result<br />
73 WMD (fixed) –1.00 (–1.39 to –0.61)*<br />
HAQ, mean change from baseline – 0 0 Not estimable Data not available<br />
DAS28, end <strong>of</strong> study result – 0 0 Not estimable Data not available<br />
Modified van de Heijde–Sharp score,<br />
mean change from baseline<br />
– 0 0 Not estimable Data not available<br />
Withdrawal for any reasons vs MTX 1137 58 RR (fixed) 0.48 (0.25 to 0.93)*<br />
Withdrawal due to lack <strong>of</strong> efficacy vs MTX 1137 58 RR (fixed) 0.32 (0.15 to 0.69)*<br />
Withdrawal due to adverse events vs MTX 1137 58 RR (fixed) 3.00 (0.17 to 52.53)<br />
Death – 0 0 Not estimable Data not available<br />
SAEs – 0 0 Not estimable Data not available<br />
Malignancy vs MTX 1137 58 Not estimable No events<br />
Serious infection vs MTX 1137 58 Not estimable No events<br />
Any infection vs placebo 1136 73 RR (fixed) 2.94 (0.37 to 23.06)<br />
* Statistically significant result (p < 0.05).<br />
151