A systematic review of the effectiveness of adalimumab
A systematic review of the effectiveness of adalimumab
A systematic review of the effectiveness of adalimumab
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
following properties, according to <strong>the</strong> values <strong>of</strong> L<br />
and U:<br />
● U < 5000 or L > 200,000: U/L < 2.5<br />
● U < 10,000 or L > 100,000: U/L < 2.0<br />
● U < 20,000 or L > 50,000: U/L < 1.5<br />
● U < 30,000 or L > 30,000: U/L < 1.2<br />
● L < 30,000 and U > 30,000: U/L < 1.1.<br />
In cases where <strong>the</strong>re were more than two options<br />
to compare, <strong>the</strong> more important comparisons are<br />
those between an option including a TNF<br />
inhibitor and <strong>the</strong> baseline without that TNF<br />
inhibitor. These are referred to as ‘major<br />
comparisons’. Comparisons between different<br />
strategies including TNF inhibitors are referred to<br />
as ‘minor comparisons’. Results are given for <strong>the</strong><br />
following minor comparisons:<br />
● effect <strong>of</strong> adding methotrexate (<strong>adalimumab</strong> plus<br />
methotrexate versus <strong>adalimumab</strong> alone,<br />
etanercept plus methotrexate versus etanercept<br />
alone)<br />
● comparison between mono<strong>the</strong>rapies<br />
(<strong>adalimumab</strong> versus etanercept alone)<br />
● comparison between combinations with<br />
methotrexate (<strong>adalimumab</strong> plus methotrexate<br />
versus etanercept plus methotrexate versus<br />
infliximab plus methotrexate).<br />
The model was first run with 10,000 patients. If<br />
any major comparison gave insufficiently precise<br />
results, <strong>the</strong>n <strong>the</strong> number <strong>of</strong> patients was increased<br />
to 20,000, <strong>the</strong>n to 40,000, <strong>the</strong>n to 100,000, <strong>the</strong>n<br />
to 200,000, and so on as necessary until all major<br />
comparisons gave sufficiently precise results. If any<br />
quoted minor comparison was insufficiently<br />
precise at this stage, <strong>the</strong> number <strong>of</strong> patients was<br />
increased once more. The actual number <strong>of</strong><br />
patients modelled in each case is stated.<br />
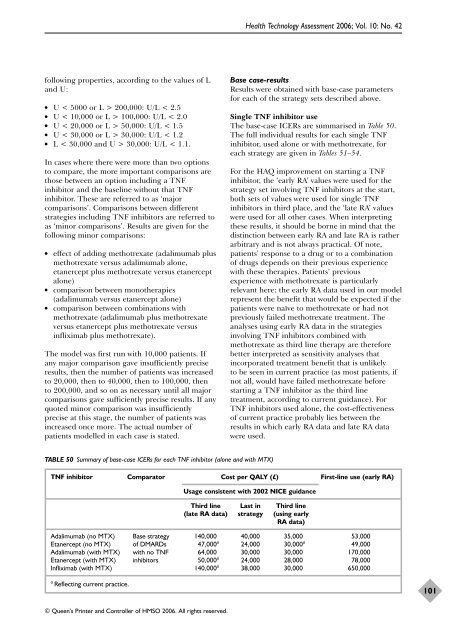
TABLE 50 Summary <strong>of</strong> base-case ICERs for each TNF inhibitor (alone and with MTX)<br />
© Queen’s Printer and Controller <strong>of</strong> HMSO 2006. All rights reserved.<br />
Health Technology Assessment 2006; Vol. 10: No. 42<br />
TNF inhibitor Comparator Cost per QALY (£) First-line use (early RA)<br />
Usage consistent with 2002 NICE guidance<br />
Third line Last in Third line<br />
(late RA data) strategy (using early<br />
RA data)<br />
Adalimumab (no MTX) Base strategy 140,000 40,000 35,000 53,000<br />
Etanercept (no MTX) <strong>of</strong> DMARDs 47,000 a 24,000 30,000 a 49,000<br />
Adalimumab (with MTX) with no TNF 64,000 30,000 30,000 170,000<br />
Etanercept (with MTX) inhibitors 50,000 a 24,000 28,000 78,000<br />
Infliximab (with MTX) 140,000 a 38,000 30,000 650,000<br />
a Reflecting current practice.<br />
Base case-results<br />
Results were obtained with base-case parameters<br />
for each <strong>of</strong> <strong>the</strong> strategy sets described above.<br />
Single TNF inhibitor use<br />
The base-case ICERs are summarised in Table 50.<br />
The full individual results for each single TNF<br />
inhibitor, used alone or with methotrexate, for<br />
each strategy are given in Tables 51–54.<br />
For <strong>the</strong> HAQ improvement on starting a TNF<br />
inhibitor, <strong>the</strong> ‘early RA’ values were used for <strong>the</strong><br />
strategy set involving TNF inhibitors at <strong>the</strong> start,<br />
both sets <strong>of</strong> values were used for single TNF<br />
inhibitors in third place, and <strong>the</strong> ‘late RA’ values<br />
were used for all o<strong>the</strong>r cases. When interpreting<br />
<strong>the</strong>se results, it should be borne in mind that <strong>the</strong><br />
distinction between early RA and late RA is ra<strong>the</strong>r<br />
arbitrary and is not always practical. Of note,<br />
patients’ response to a drug or to a combination<br />
<strong>of</strong> drugs depends on <strong>the</strong>ir previous experience<br />
with <strong>the</strong>se <strong>the</strong>rapies. Patients’ previous<br />
experience with methotrexate is particularly<br />
relevant here: <strong>the</strong> early RA data used in our model<br />
represent <strong>the</strong> benefit that would be expected if <strong>the</strong><br />
patients were naïve to methotrexate or had not<br />
previously failed methotrexate treatment. The<br />
analyses using early RA data in <strong>the</strong> strategies<br />
involving TNF inhibitors combined with<br />
methotrexate as third line <strong>the</strong>rapy are <strong>the</strong>refore<br />
better interpreted as sensitivity analyses that<br />
incorporated treatment benefit that is unlikely<br />
to be seen in current practice (as most patients, if<br />
not all, would have failed methotrexate before<br />
starting a TNF inhibitor as <strong>the</strong> third line<br />
treatment, according to current guidance). For<br />
TNF inhibitors used alone, <strong>the</strong> cost-<strong>effectiveness</strong><br />
<strong>of</strong> current practice probably lies between <strong>the</strong><br />
results in which early RA data and late RA data<br />
were used.<br />
101