A systematic review of the effectiveness of adalimumab
A systematic review of the effectiveness of adalimumab
A systematic review of the effectiveness of adalimumab
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
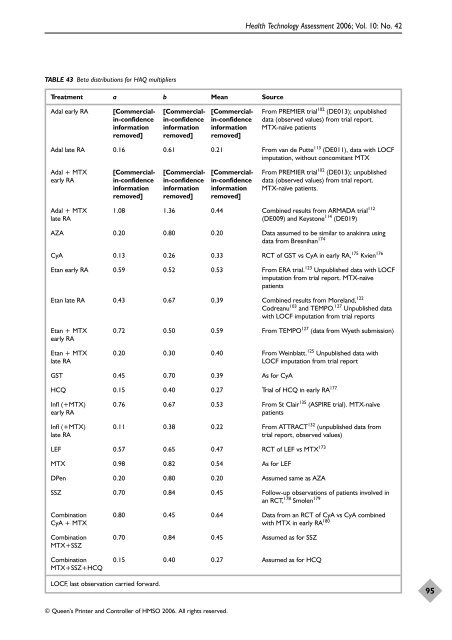
TABLE 43 Beta distributions for HAQ multipliers<br />
Treatment a b Mean Source<br />
© Queen’s Printer and Controller <strong>of</strong> HMSO 2006. All rights reserved.<br />
Health Technology Assessment 2006; Vol. 10: No. 42<br />
Adal early RA [Commercial- [Commercial- [Commercial- From PREMIER trial 102 (DE013); unpublished<br />
in-confidence in-confidence in-confidence data (observed values) from trial report.<br />
information information information MTX-naïve patients<br />
removed] removed] removed]<br />
Adal late RA 0.16 0.61 0.21 From van de Putte 113 (DE011), data with LOCF<br />
imputation, without concomitant MTX<br />
Adal + MTX [Commercial- [Commercial- [Commercial- From PREMIER trial 102 (DE013); unpublished<br />
early RA in-confidence in-confidence in-confidence data (observed values) from trial report.<br />
information information information MTX-naïve patients.<br />
removed] removed] removed]<br />
Adal + MTX 1.08 1.36 0.44 Combined results from ARMADA trial 112<br />
late RA (DE009) and Keystone 114 (DE019)<br />
AZA 0.20 0.80 0.20 Data assumed to be similar to anakinra using<br />
data from Bresnihan 174<br />
CyA 0.13 0.26 0.33 RCT <strong>of</strong> GST vs CyA in early RA, 175 Kvien 176<br />
Etan early RA 0.59 0.52 0.53 From ERA trial. 123 Unpublished data with LOCF<br />
imputation from trial report. MTX-naïve<br />
patients<br />
Etan late RA 0.43 0.67 0.39 Combined results from Moreland, 122<br />
Codreanu103 and TEMPO. 127 Unpublished data<br />
with LOCF imputation from trial reports<br />
Etan + MTX 0.72 0.50 0.59 From TEMPO 127 (data from Wyeth submission)<br />
early RA<br />
Etan + MTX 0.20 0.30 0.40 From Weinblatt. 125 Unpublished data with<br />
late RA LOCF imputation from trial report<br />
GST 0.45 0.70 0.39 As for CyA<br />
HCQ 0.15 0.40 0.27 Trial <strong>of</strong> HCQ in early RA 177<br />
Infl (+MTX) 0.76 0.67 0.53 From St Clair 135 (ASPIRE trial). MTX-naïve<br />
early RA patients<br />
Infl (+MTX) 0.11 0.38 0.22 From ATTRACT 132 (unpublished data from<br />
late RA trial report, observed values)<br />
LEF 0.57 0.65 0.47 RCT <strong>of</strong> LEF vs MTX 173<br />
MTX 0.98 0.82 0.54 As for LEF<br />
DPen 0.20 0.80 0.20 Assumed same as AZA<br />
SSZ 0.70 0.84 0.45 Follow-up observations <strong>of</strong> patients involved in<br />
an RCT, 178 Smolen 179<br />
Combination 0.80 0.45 0.64 Data from an RCT <strong>of</strong> CyA vs CyA combined<br />
CyA + MTX with MTX in early RA 180<br />
Combination 0.70 0.84 0.45 Assumed as for SSZ<br />
MTX+SSZ<br />
Combination 0.15 0.40 0.27 Assumed as for HCQ<br />
MTX+SSZ+HCQ<br />
LOCF, last observation carried forward.<br />
95