Answer Key to Assignment #2
Answer Key to Assignment #2
Answer Key to Assignment #2
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
Chem 2600<br />
<strong>Assignment</strong> 2 <strong>Answer</strong> <strong>Key</strong><br />
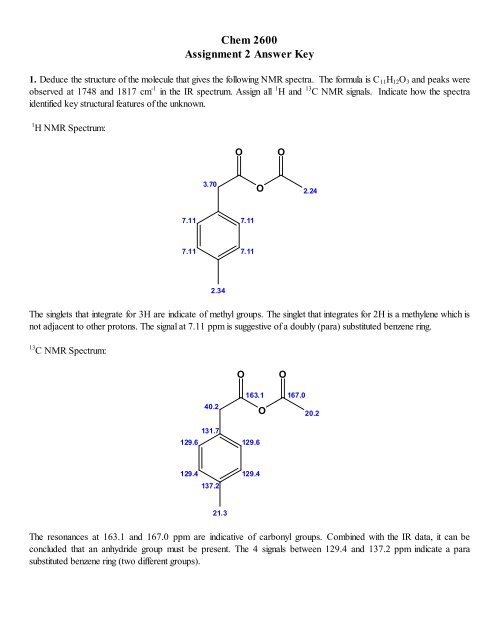
1. Deduce the structure of the molecule that gives the following NMR spectra. The formula is C 11H12O3 and peaks were<br />
observed at 1748 and 1817 cm -1 in the IR spectrum. Assign all 1 H and 13 C NMR signals. Indicate how the spectra<br />
identified key structural features of the unknown.<br />
1 H NMR Spectrum:<br />
7.11<br />
3.70<br />
7.11<br />
7.11 7.11<br />
2.34<br />
O<br />
The singlets that integrate for 3H are indicate of methyl groups. The singlet that integrates for 2H is a methylene which is<br />
not adjacent <strong>to</strong> other pro<strong>to</strong>ns. The signal at 7.11 ppm is suggestive of a doubly (para) substituted benzene ring.<br />
13 C NMR Spectrum:<br />
129.6<br />
129.4<br />
40.2<br />
131.7<br />
137.2<br />
21.3<br />
O<br />
129.6<br />
129.4<br />
O<br />
O<br />
163.1 167.0<br />
The resonances at 163.1 and 167.0 ppm are indicative of carbonyl groups. Combined with the IR data, it can be<br />
concluded that an anhydride group must be present. The 4 signals between 129.4 and 137.2 ppm indicate a para<br />
substituted benzene ring (two different groups).<br />
O<br />
O<br />
2.24<br />
20.2
2. Match the 1 H NMR spectra (1-4) with the correct chemical structure (A-D).<br />
Spectrum 1: A. The aromatic region is indicative of a meta substituted benzene ring (two same groups). The presence of<br />
only one septet and one doublet indicated these groups must be the same as well.<br />
Spectrum 2: C. The aromatic region is indicative of a para substituted benzene ring (two same groups). The septet<br />
appears as 2.6 ppm, which agrees with the following environment: H – Csp 3 – Csp 2 .<br />
Spectrum 3: B. The aromatic region contains several overlapping signals. Clearly this must be one of the two meta<br />
substituted benzene rings (A or B). The two different septets and doublets indicates that the two groups bound <strong>to</strong> the<br />
benzene ring are different.<br />
Spectrum 4: D. The aromatic region is indicative of a para substituted benzene ring (two same groups). The septet<br />
appears as 5.3 ppm, which agrees with the following environment: H – Csp 3 – O – Csp 2 .<br />
3. In the 1 H NMR spectra of the following two compounds, HA appears as a 1:2:1 triplet, but HB appears as a double of<br />
doublets. Briefly explain why this is the case.<br />
HA has the same magnitude of J coupling <strong>to</strong> each adjacent pro<strong>to</strong>n. Thus, it appears as a 1:2:1 triplet (n+1 rule applies).<br />
HB has a different sized J coupling ( 3 J and 4 J) <strong>to</strong> each of the other two pro<strong>to</strong>ns on the benzene ring. This causes a doublet<br />
of doublets as explained by the following tree diagram.
4. In the 60 MHz spectrum of a certain compound there are two signals separated by 2 ppm.<br />
i) What is this separation in Hz?<br />
2 ppm (Hz/MHz) x 60 MHz = 120 Hz<br />
ii) In a 250 MHz instrument, what is the separation between these signals in ppm?<br />
2 ppm<br />
iii) In a 250 MHz instrument, what is the separation between these signals in Hz?<br />
500 Hz<br />
5. For each of the following spectra, determine the structure.<br />
i)<br />
7<br />
C14H14O. Relative integration is 5:2.<br />
6<br />
5<br />
4<br />
PPM<br />
3<br />
O<br />
2<br />
1<br />
0
ii)<br />
C5H12O. Relative integration is 2:1:1:2:6.<br />
iii)<br />
10<br />
C9H10O3. Relative integration 1:2:2:2:3.<br />
3<br />
8<br />
2<br />
PPM<br />
6<br />
PPM<br />
6. How many pro<strong>to</strong>n signals, and how many carbon signals would you expect from each of the following molecules?<br />
A) 3C and 4H<br />
B) 4C and 3H<br />
C) 5C and 6H<br />
OH<br />
O O H<br />
O CH 2CH 3<br />
4<br />
1<br />
2<br />
0<br />
0
7. You wish <strong>to</strong> carry out the following reactions, and you have the 1 H NMR spectrum of each starting material. In each<br />
case explain how the NMR spectrum of the product and starting material would be expected <strong>to</strong> differ.<br />
a) The spectrum would simplify from two signals <strong>to</strong> one.<br />
b) There would be a slight upfield shift of the lone signal.<br />
8. How would you distinguish between the compounds within each set below using their 1 H NMR spectra? Explain<br />
carefully, briefly and explicitly what features of the NMR spectra you would use.<br />
a) Due <strong>to</strong> its symmetry, the first compound has only 3 signals while the second has 6 signals.<br />
b) The first has two singlets while the second will have a singlet, a doublet and a multiplet.<br />
c) All three will exhibit two doublets in the downfield vinyl region. The coupling constant, however, will be<br />
approximately 10 Hz in the first case, 16 Hz in the second and 2 Hz in the last case.<br />
9. There are four isomers with the formula C 4 H 9 Cl. The pro<strong>to</strong>n-decoupled 13 C NMR spectrum of one of the isomers is<br />
shown below.<br />
i) Of the four possible isomers, which might give this spectrum?<br />
The four isomers are:<br />
Cl<br />
Cl<br />
CH2Cl<br />
Of these, only the first and last will exhibit four carbon signals. The second compound will have only two; the<br />
third three.<br />
ii) How would the pro<strong>to</strong>n-coupled 13 C NMR spectrum help you decide which isomer it is?<br />
The pro<strong>to</strong>n coupled spectrum will give quartets for methyls, triplets for methylenes and doublets for methine<br />
pro<strong>to</strong>ns. Thus, the pro<strong>to</strong>n coupled spectrum for the first compound will have one quartet and three triplets while<br />
the last compound will have two quartets, a doublet and a triplet.<br />
Cl