Answers to PS #1 - Classes at U. of L.
Answers to PS #1 - Classes at U. of L. Answers to PS #1 - Classes at U. of L.
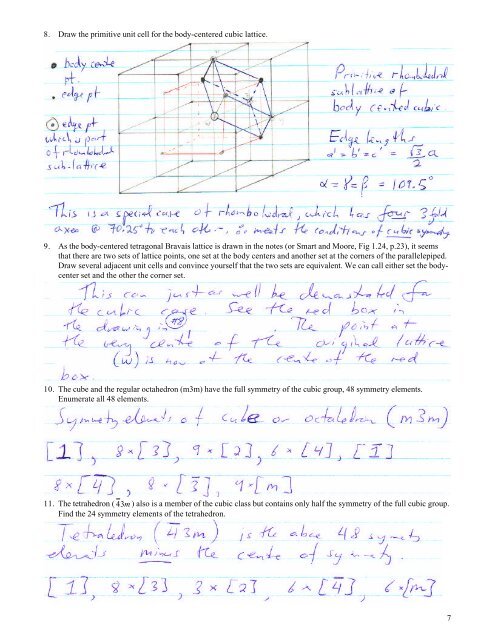
8. Draw the primitive unit cell for the body-centered cubic lattice. 9. As the body-centered tetragonal Bravais lattice is drawn in the notes (or Smart and Moore, Fig 1.24, p.23), it seems that there are two sets of lattice points, one set at the body centers and another set at the corners of the parallelepiped. Draw several adjacent unit cells and convince yourself that the two sets are equivalent. We can call either set the bodycenter set and the other the corner set. 10. The cube and the regular octahedron (m3m) have the full symmetry of the cubic group, 48 symmetry elements. Enumerate all 48 elements. 11. The tetrahedron ( 43m ) also is a member of the cubic class but contains only half the symmetry of the full cubic group. Find the 24 symmetry elements of the tetrahedron. 7
12. a) Show the interrelationship between the cube and the tetrahedron (with clear sketches). b) Show the interrelationship between the cube and the octahedron. 13. a) The contents of the unit cell of any compound must contain an integral number of formula units. Why? b) Note that unit cell boundaries "slice" atoms into fragments: An atom on a face will be split in half between two cells; one on an edge will be split into quarters among four cells, etc. Identify the number of Na + and Cl - ions in the unit cell of sodium chloride and state how many formula units of NaCl the unit cell contains. The unit cell of NaCl is illustrated, e.g. in Smart and Moore, p. 31. c) Why is the unit cell of NaCl so large? Why can a smaller unit not be chosen? Demonstrate this by trying it. 8
- Page 1: Chemistry 4000 Problem set #1 Answe
- Page 4: 6. Draw the following Bravais latti
- Page 7: 8. Draw the primitive unit cell for
- Page 12: 16. As was done in class, assign th
8. Draw the primitive unit cell for the body-centered cubic l<strong>at</strong>tice.<br />
9. As the body-centered tetragonal Bravais l<strong>at</strong>tice is drawn in the notes (or Smart and Moore, Fig 1.24, p.23), it seems<br />
th<strong>at</strong> there are two sets <strong>of</strong> l<strong>at</strong>tice points, one set <strong>at</strong> the body centers and another set <strong>at</strong> the corners <strong>of</strong> the parallelepiped.<br />
Draw several adjacent unit cells and convince yourself th<strong>at</strong> the two sets are equivalent. We can call either set the bodycenter<br />
set and the other the corner set.<br />
10. The cube and the regular octahedron (m3m) have the full symmetry <strong>of</strong> the cubic group, 48 symmetry elements.<br />
Enumer<strong>at</strong>e all 48 elements.<br />
11. The tetrahedron ( 43m ) also is a member <strong>of</strong> the cubic class but contains only half the symmetry <strong>of</strong> the full cubic group.<br />
Find the 24 symmetry elements <strong>of</strong> the tetrahedron.<br />
7