ORNL-1816 - the Molten Salt Energy Technologies Web Site
ORNL-1816 - the Molten Salt Energy Technologies Web Site ORNL-1816 - the Molten Salt Energy Technologies Web Site
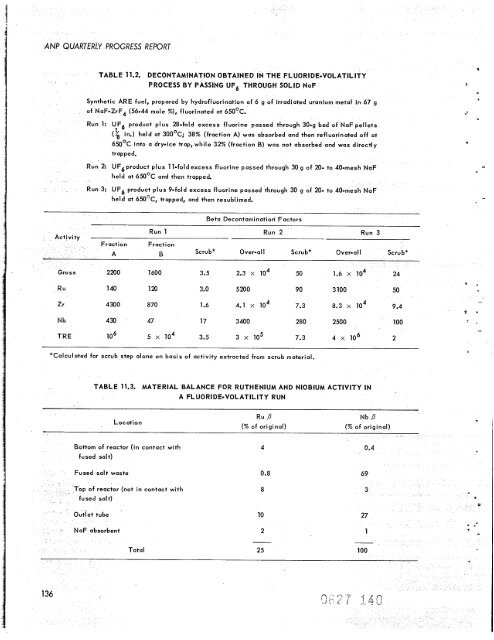
ANP QUARTERLY PROGRESS REPORT 136 Activity TABLE 11.2. DECONTAMINATION OBTAINED IN THE FLUORIDE-VOLATILITY PROCESS BY PASSING UF6 THROUGH SOLID NaF Synthetic ARE fuel, prepored by hydrofluorinotion of 6 g of irradiated uranium metal in 67 g of NaF-ZrF4 (56-44 mole %), fluorinated at 65OOC. Run 1: UF6 product plus 28-fold excess fluorine passed through 30-9 bed of NaF pellets (k in.) held at 30OoC; 38% (fraction A) was absorbed and then refluorinated off at 650°C into o dry-ice trap, while 32% (fraction B) was not absorbed and was directly trapped. Run 2: UF6product plus 11-foldexcess fluorine passed through 30g of 20- to 40-mesh held at 65OoC and then trapped. Run 3: uF6 product plus 9-fold excess fluorine passed through 30 g of 20- to 40-mesh NaF held at 650°C, tropped, and then resublimed. Beta Decontamination’ Factors Run 1 Run 2 Run 3 Ru 140 120 3.0 5200 90 3 100 50 Zr 4300 8 70 1.6 4.1 lo4 7.3 8.3 lo4 9.4 Nb 430 47 17 3400 280 2500 100 TRE lo6 5 lo4 3.5 3 lo5 7.3 4 x lo6 2 *Calculated for scrub step alone on basis of activity extracted from scrub material. TABLE 11.3. MATERIAL BALANCE FOR RUTHENIUM AND NIOBIUM ACTIVITY IN A FLUORIDE-VOLATILITY RUN Location Bottom of reactor (in contact with fused salt) Fused salt waste op of reactor (not in contact with NoF absorbent Ru P Nb P (% of original) (% of original) 4 0.8 8 10 2 - 0.4 69 3 27 1
,. % I i s * ruthenium was apparently volatilized out of the reactor entirely. Ruthenium and niobium plated out heavily on the metal walls of the reactor. The distribution ratio between metal wall and salt was about 5 for ruthenium activity and less than 0.01 for niobium activity. The behavior of plutonium in the fused salt fluoride-volatil ity process is important in processing fuel that has a high proportion of U238. The results of many fluorination runs at 650°C show that only about 0.01% of the plutonium is carried over with the UF, product. Further study was carried out on the absorption of UF, in NaF-ZrF, (56-44 mole %) at 65OOC. In one trial, 9 g of UF, was absorbed in 30 g of NaF-ZrF, in 1 hr with no noticeable loss. However, passage of helium through the fused salt, either concurrently with or after the UF, addition, erable fuming. A positive test for fluorine with potassium iodide paper was obtained, which indicated reduction of the adsorbed UF, to the tetra- or pentavalent form of uranium. A gravimetric test, based on the reaction of fluorine with sodium chloride, showed that the breakdown of UF, to F, and UF, amounts to 1 to 2% per hour at 65OOC. Engineering information is needed on two steps in the fluoride-volatility process: fluorination and ,. UF, cold-trapping. Effective contacting of fluorine with molten ARE fuel is desirable to minimize fluorine consumption and gaseous waste and to assure complete recovery of the uranium. The cold trap should be operated in such a manner that all the UF, will be condensed and collected on the PERIOD ENDING DECEMBER 70,7954 properties of the liquid could not be demonstrated by a simple correlation of friction factor with Reynolds number, such as is found in ordinary fluid flow through pipes. The acetylerie tetra- bromide studies showed that vertical mixirig of the liquid phase was induced by a gas rate of 9.5 cfm in a 12-in.-dia column. Equipment is now being assembled to verify these conclusions with molten Na F-Z r F ,. Cold traps to be used for a quantitative study of the effect of temperature and gas flow rate on the completeness of UF, removal are being con- structed. The design of these traps is based on a K-25 cold-trap design. APPLICATIONS OF FUSED SALT-VOLATILITY PROCESSES A long-range study has been made to survey the over-all feasibility of fused salt-voIatiIi,ty tech- niques in the chemical processing of ARE-type reactor fuels and certain types of heterogeneous reactor fuel elements. The volumes of raclioactive waste from such processes should be mulch lower than those from the aqueous processes, and processing costs should be low, even though a means of disposing of excess fluorine will have to be provided. Total chemical costs, ?which in present aqueous processes represent approximately 10% of operating costs, have been estimated to be 20g per gram of U235. The operational procedure is much simpler, and the equipment should be inexpensive, even though nickel will be required as the material of construction. Results of recent work on fuel element dissolution 137
- Page 98 and 99: 1 I I I . . ANP QUARTERLY PROGRESS
- Page 100 and 101: ANP QUARTERLY PROGRESS REPORT Fig.
- Page 102 and 103: 06 dWnd lV'91NVH33W 01 A XI13 X3tlA
- Page 104 and 105: ANP QUARTERLY PROGRESS REPORT 0.001
- Page 106 and 107: I ANP QUARTERLY PROGRESS REPORT ] 1
- Page 108 and 109: ANP QUARTERLY PROGRESS REPORT If a
- Page 110 and 111: ANP QUARTERLY PROGRESS REPORT phase
- Page 112 and 113: ANP QUARTERLY PROGRESS REPORT 7. ME
- Page 114 and 115: ANP QUARTERLY PROGRESS REPORT fabri
- Page 116 and 117: ANP QUARTERLY PROGRESS REPORT - N -
- Page 118 and 119: ANP QUARTERLY PROGRESS REPORT Fig.
- Page 120 and 121: 1 I ANP QUARTERLY PROGRESS REPORT h
- Page 122 and 123: ANP QUARTERLY PROGRESS REPORT rruga
- Page 124 and 125: ANP QUARTERLY PROGRESS REPORT eat t
- Page 126 and 127: ANP QUARTERLY PROGRESS REPORT Plast
- Page 128 and 129: ANP QUARTERLY PROGRESS REPORT 0.2 0
- Page 130 and 131: ANP QUARTERLY PROGRESS REPORT STAIN
- Page 132 and 133: i ANP QUARTERLY PROGRESS REPORT The
- Page 134 and 135: ANP QUARTERLY PROGRESS REPORT Three
- Page 136 and 137: ANP QUARTERLY PROGRESS REPORT - ORN
- Page 138 and 139: ANP QUARTERLY PROGRESS REPORT kw at
- Page 140 and 141: ANP QUARTERLY PROGRESS REPORT felt
- Page 142 and 143: ANP QUARTERLY PROGRESS REPORT chlor
- Page 144 and 145: ANP QUARTERLY PROGRESS REPORT prese
- Page 146 and 147: ! ANP QUARTERLY PROGRESS REPORT 11.
- Page 150 and 151: ANP QUARTERLY PROGRESS REPORT stand
- Page 152 and 153: fused salt bath to permit dissoluti
- Page 154 and 155: Fr . F
- Page 156 and 157: ANP QUARlERlY PROGRESS REPORT chara
- Page 158 and 159: ANP QUARTERLY PROGRESS REPORT injur
- Page 160 and 161: ANP QUARTERLY PROGRESS REPORT 148 X
- Page 162 and 163: ANP QUARTERLY PROGRESS REPORT In co
- Page 164 and 165: ANP QUARTERLY PROGRESS REPORT - 103
- Page 166 and 167: ANP QUARTERLY PROGRESS REPORT condu
- Page 168 and 169: ANP QUARTERLY PROGRESS REPORT For m
- Page 170 and 171: 15. TOWER SHIELDING FACILITY C. E.
- Page 172 and 173: ANP QUARTERLY PROGRESS REPORT - .-
- Page 174 and 175: ANP QUARTERLY PROGRESS REPORT 162 -
- Page 176 and 177: h .e 0 c 0 L ’c v c t 0 W v) 0 a
- Page 178 and 179: f r e
ANP QUARTERLY PROGRESS REPORT<br />
136<br />
Activity<br />
TABLE 11.2. DECONTAMINATION OBTAINED IN THE FLUORIDE-VOLATILITY<br />
PROCESS BY PASSING UF6 THROUGH SOLID NaF<br />
Syn<strong>the</strong>tic ARE fuel, prepored by hydrofluorinotion of 6 g of irradiated uranium metal in 67 g<br />
of NaF-ZrF4 (56-44 mole %), fluorinated at 65OOC.<br />
Run 1: UF6 product plus 28-fold excess fluorine passed through 30-9 bed of NaF pellets<br />
(k in.) held at 30OoC; 38% (fraction A) was absorbed and <strong>the</strong>n refluorinated off at<br />
650°C into o dry-ice trap, while 32% (fraction B) was not absorbed and was directly<br />
trapped.<br />
Run 2: UF6product plus 11-foldexcess fluorine passed through 30g of 20- to 40-mesh<br />
held at 65OoC and <strong>the</strong>n trapped.<br />
Run 3: uF6 product plus 9-fold excess fluorine passed through 30 g of 20- to 40-mesh NaF<br />
held at 650°C, tropped, and <strong>the</strong>n resublimed.<br />
Beta Decontamination’ Factors<br />
Run 1 Run 2 Run 3<br />
Ru 140 120 3.0 5200 90 3 100 50<br />
Zr 4300 8 70 1.6 4.1 lo4 7.3 8.3 lo4 9.4<br />
Nb 430 47 17 3400 280 2500 100<br />
TRE lo6 5 lo4 3.5 3 lo5 7.3 4 x lo6 2<br />
*Calculated for scrub step alone on basis of activity extracted from scrub material.<br />
TABLE 11.3. MATERIAL BALANCE FOR RUTHENIUM AND NIOBIUM ACTIVITY IN<br />
A FLUORIDE-VOLATILITY RUN<br />
Location<br />
Bottom of reactor (in contact with<br />
fused salt)<br />
Fused salt waste<br />
op of reactor (not in contact with<br />
NoF absorbent<br />
Ru P Nb P<br />
(% of original) (% of original)<br />
4<br />
0.8<br />
8<br />
10<br />
2<br />
-<br />
0.4<br />
69<br />
3<br />
27<br />
1