ORNL-1771 - Oak Ridge National Laboratory
ORNL-1771 - Oak Ridge National Laboratory
ORNL-1771 - Oak Ridge National Laboratory
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
ANP QUARTERLY PROGRESS REPORT<br />
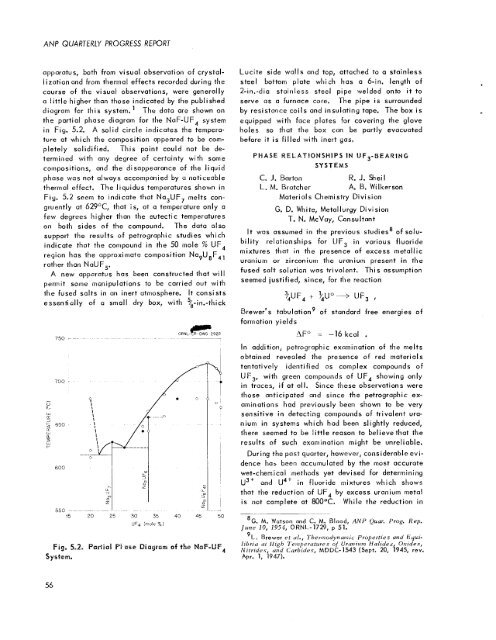
apparatus, both from visual observation of crystal-<br />
lization and from thermal effects recorded during the<br />
course of the vi sua1 observations, were generally<br />
a little higher than those indicated by the published<br />
diagram for this system.' The data are shown on<br />
the partial phase diagram for the NaF-UF, system<br />
in Fig. 5.2. A solid circle indicates the tempera-<br />
ture at which the composition appeared to be com-<br />
pletely solidified. This point could not be de-<br />
termined with any degree of certainty with some<br />
compositions, uncl the disoppeorunce of the liquid<br />
phase wus not always accompanied by B noticeable<br />
thermal effect. The liquidus temperatures shown in<br />
Fiy. 5.2 seem to indicate that Na,UF, melts con-<br />
gruently at 629OC, that is, at a temperature only u<br />
few degrees higher than the eutectic temperatures<br />
on both sides of the compound. The data also<br />
support the results of petrographic studies which<br />
indicate that the compound in the 50 mole % UF,<br />
region has the approximate composition Na,U,F,<br />
rather than NaUF,.<br />
A new appnratus has been constructed that will<br />
permit some manipulations to be carried out with<br />
the fused salts in an inert utnwsphere. It consists<br />
essentially of a small dry box, with i-in.-thick<br />
750<br />
700 ~<br />
600<br />
550 ~<br />
15 20 25 30 35 40<br />
UF4 hole %)<br />
<strong>ORNL</strong> 922<br />
Fig. 5.2. Por4ial Wase Diagram of the NaF-UF,<br />
SyeBcm.<br />
54<br />
Lucite side walls and top, attached to a stainless<br />
steel bottom plate which has a &in. length of<br />
2-in.-dia stainless steel pipe welded onto it to<br />
serve CIS a furnace core. The pipe is surrounded<br />
by resistance coils and insulating tape. The box is<br />
equipped with face plates for covering the glove<br />
holes so that the box can be partly evacuated<br />
before it is filled with inert gas,<br />
PHASE REhATlONSHlPS IN UF3-5EARING<br />
SYSTEMS<br />
C. J. Barton<br />
R. J. SheiI<br />
L. M. Brntcher<br />
A. B. Wilkerson<br />
Materials Chemistry Division<br />
G. D. White, Metallurgy Division<br />
T. N. McVay, Consultant<br />
It was assuined in the previous studies* of solu-<br />
bility relationships for IJF, in various fluoride<br />
mixtures that in the presence of excess metallic<br />
uranium or zirconium the uranium present in the<br />
fused salt solution WQS trivalent, This assumption<br />
seemed justified, since, for the reaction<br />
Brewer's tabulation' of standard free energies of<br />
formation yields<br />
AF" = -16 kcal .<br />
In addition, petrographic examination of the melts<br />
obtained revealed the presence of red materials<br />
tentatively identified as complex compounds of<br />
UF,, with green compounds of UF, showing only<br />
in traces, if at all. Since these observations were<br />
those anticipated and since the petrographic ex-<br />
ominations had previously been shown to be very<br />
sensitive in detecting compounds of trivalent ura-<br />
nium in systems which had been slightly reduced,<br />
there seemed to be little reason to believe that the<br />
results of such examination might be unreliable.<br />
During the past quarter, however, considerable evi-<br />
dence ha:, been accumulated by the most accurate<br />
wet-chemicol methods yet devised for determining<br />
U3+ and U4' in fluoride mixtures which shows<br />
that the reduction of UF, by excess uranium metal<br />
is not complete at 800°C. While the reduction in<br />
*G. M. Watson and C. Ah. Blood, ANP @UT. Prog. Rep.<br />
lune IO, 1954, QHNL-1729, p 51.<br />
'L, Brewer et ul., Thenodynarfiic Properties and Eyui-<br />
libria at High Temperalures of Uranium Halides, Oxides,<br />
Nitrides, urd Carbides, MDDC-1543 (Sept. 20, 1945, rev.<br />
Apr. 1, 11947).


![Review of Molten Salt Reactor Physics Calculations [Disc 2]](https://img.yumpu.com/21979492/1/190x247/review-of-molten-salt-reactor-physics-calculations-disc-2.jpg?quality=85)













