ORNL-2106 - the Molten Salt Energy Technologies Web Site
ORNL-2106 - the Molten Salt Energy Technologies Web Site
ORNL-2106 - the Molten Salt Energy Technologies Web Site
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
ANP PROJECT PROGRESS REPORT<br />
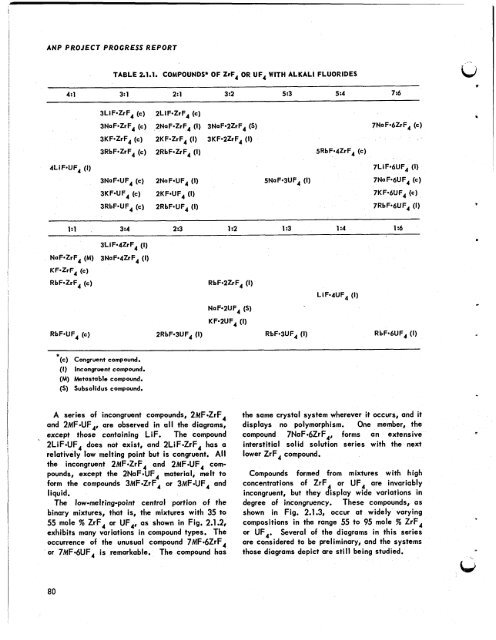
TABLE 2.1.1. COMPOUNDS* OF ZrF, OR UF, WITH ALKALI FLUORIDES<br />
4:l 3: 1 2:l 3:2 5:3 5 :4 7:6<br />
~~ ~<br />
3LiF*ZrF4 (c) 2LiF*ZrF4 (c)<br />
3NoF*ZrF4 (c) 2NaF-ZrF, (I) 3NoF-2ZrF4 (S) 7Na F*6Zr F, (c)<br />
3KF-ZrF4 (c) 2KF=ZrF4 (I) 3KF*2ZrF4 (I)<br />
3RbF*ZrF4 (c) 2RbF-ZrF4 (I) 5RbF*4ZrF4 (c)<br />
4LiF*UF, (I) 7LiF*6UF4 (I)<br />
3NaF*UF4 (c) 2NaF*UF4 (I) 5NoF*3UF4 (I) 7NoF*6UF4 (c)<br />
3KF*UF4 (c) 2KF*UF4 (I) 7KF*6UF4 (c)<br />
3RbF*UF4 (c) 2RbF*UF4 (I) 7RbF*6UF4 (I)<br />
1:l 3:4 2:3 1 :2 1 :3 1 :4 1 :6<br />
3LiF*4ZrF4 (I)<br />
NaF-ZrF, (M) 3NaF*4ZrF4 (I)<br />
KF-ZrF, (c)<br />
RbF*ZrF, (c) RbF*2ZrF4 (I)<br />
NaF*2UF4 (S)<br />
KF*2UF4 (I)<br />
LiF-dlJF, (I)<br />
RbF-UF, (c) 2RbF*3UF4 (I) RbF*3UF4 (I) RbF*6UF4 (I)<br />
t<br />
(c) Congruent compound.<br />
(I) Incongruent compound.<br />
(M) Metastable compound.<br />
(S) Subsolidus compound.<br />
A series of incongruent compounds, 2MF*ZrF,<br />
and 2MF*UF,, are observed in all <strong>the</strong> diagrams,<br />
except those containing LiF. The compound<br />
2LiF*UF, does not exist, and 2LiF*ZrF4 has a<br />
relatively low melting point but is congruent. All<br />
<strong>the</strong> incongruent 2MF*ZrF, and 2MF*UF, com-<br />
pounds, except <strong>the</strong> 2NaF*UF4 material, melt to<br />
form <strong>the</strong> compounds 3MF*ZrF4 or 3MF*UF, and<br />
liquid.<br />
The low-melting-point central portion of <strong>the</strong><br />
binary mixtures, that is, <strong>the</strong> mixtures with 35 to<br />
55 mole % ZrF, or UF, as shown in Fig. 2.1.2,<br />
exhibits many variations in compound types. The<br />
occurrence of <strong>the</strong> unusual compound 7MF.6ZrF4<br />
or 7MF*6UF4 is remarkable. The compound has<br />
80<br />
<strong>the</strong> same crystal system wherever it occurs, and it<br />
displays no polymorphism. One member, <strong>the</strong><br />
compound 7NaF*6ZrF4, forms an extensive<br />
interstitial solid solution series with <strong>the</strong> next<br />
lower ZrF, compound.<br />
Compounds formed from mixtures with high<br />
concentrations of ZrF, or UF, are invariably<br />
incongruent, but <strong>the</strong>y display wide variations in<br />
degree of incongruency. These compounds, as<br />
shown in Fig. 2.1.3, occur at widely varying<br />
compositions in <strong>the</strong> range 55 to 95 mole % ZrF,<br />
or UF,. Several of <strong>the</strong> diagrams in this series<br />
are considered to be preliminary, and <strong>the</strong> systems<br />
those diagrams depict are still being studied.<br />
-<br />
G<br />
S<br />
a<br />
c


![Review of Molten Salt Reactor Physics Calculations [Disc 2]](https://img.yumpu.com/21979492/1/190x247/review-of-molten-salt-reactor-physics-calculations-disc-2.jpg?quality=85)













