Corrosion (presentation)
Corrosion (presentation)
Corrosion (presentation)
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
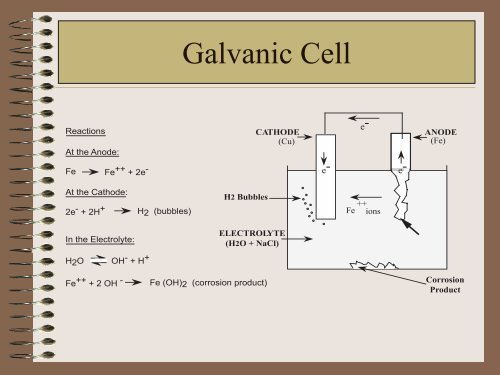
Reactions<br />
At the Anode:<br />
Fe Fe ++ + 2e -<br />
At the Cathode:<br />
2e - + 2H + H2 (bubbles)<br />
In the Electrolyte:<br />
H2O OH - + H +<br />
Galvanic Cell<br />
H2 Bubbles<br />
CATHODE<br />
(Cu)<br />
ELECTROLYTE<br />
(H2O + NaCl)<br />
Fe ++ + 2 OH - Fe (OH)2 (corrosion product)<br />
-<br />
e<br />
-<br />
e<br />
Fe ++ ions<br />
-<br />
e<br />
ANODE<br />
(Fe)<br />
<strong>Corrosion</strong><br />
Product