A solution and solid state study of niobium complexes University of ...
A solution and solid state study of niobium complexes University of ...
A solution and solid state study of niobium complexes University of ...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
Chapter 5<br />
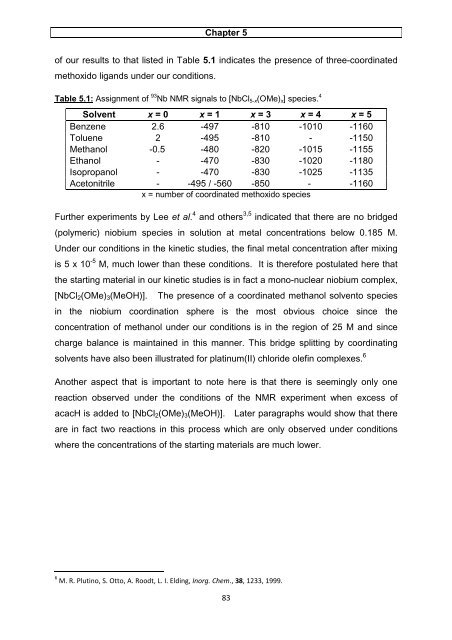
<strong>of</strong> our results to that listed in Table 5.1 indicates the presence <strong>of</strong> three-coordinated<br />
methoxido lig<strong>and</strong>s under our conditions.<br />
Table 5.1: Assignment <strong>of</strong> 93 Nb NMR signals to [NbCl5-x(OMe)x] species. 4<br />
Solvent x = 0 x = 1 x = 3 x = 4 x = 5<br />
Benzene 2.6 -497 -810 -1010 -1160<br />
Toluene 2 -495 -810 - -1150<br />
Methanol -0.5 -480 -820 -1015 -1155<br />
Ethanol - -470 -830 -1020 -1180<br />
Isopropanol - -470 -830 -1025 -1135<br />
Acetonitrile - -495 / -560 -850 - -1160<br />
x = number <strong>of</strong> coordinated methoxido species<br />
Further experiments by Lee et al. 4 <strong>and</strong> others 3,5 indicated that there are no bridged<br />
(polymeric) <strong>niobium</strong> species in <strong>solution</strong> at metal concentrations below 0.185 M.<br />
Under our conditions in the kinetic studies, the final metal concentration after mixing<br />
is 5 x 10 -5 M, much lower than these conditions. It is therefore postulated here that<br />
the starting material in our kinetic studies is in fact a mono-nuclear <strong>niobium</strong> complex,<br />
[NbCl2(OMe)3(MeOH)]. The presence <strong>of</strong> a coordinated methanol solvento species<br />
in the <strong>niobium</strong> coordination sphere is the most obvious choice since the<br />
concentration <strong>of</strong> methanol under our conditions is in the region <strong>of</strong> 25 M <strong>and</strong> since<br />
charge balance is maintained in this manner. This bridge splitting by coordinating<br />
solvents have also been illustrated for platinum(II) chloride olefin <strong>complexes</strong>. 6<br />
Another aspect that is important to note here is that there is seemingly only one<br />
reaction observed under the conditions <strong>of</strong> the NMR experiment when excess <strong>of</strong><br />
acacH is added to [NbCl2(OMe)3(MeOH)]. Later paragraphs would show that there<br />
are in fact two reactions in this process which are only observed under conditions<br />
where the concentrations <strong>of</strong> the starting materials are much lower.<br />
6 M. R. Plutino, S. Otto, A. Roodt, L. I. Elding, Inorg. Chem., 38, 1233, 1999.<br />
83