A solution and solid state study of niobium complexes University of ...
A solution and solid state study of niobium complexes University of ... A solution and solid state study of niobium complexes University of ...
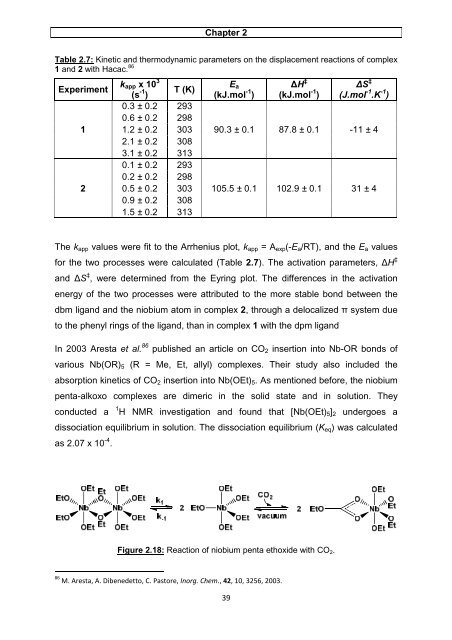
Chapter 2 Table 2.7: Kinetic and thermodynamic parameters on the displacement reactions of complex 1 and 2 with Hacac. 86 Experiment kapp x 10 3 1 2 (s -1 ) T (K) 0.3 ± 0.2 293 0.6 ± 0.2 298 1.2 ± 0.2 303 2.1 ± 0.2 308 3.1 ± 0.2 313 0.1 ± 0.2 293 0.2 ± 0.2 298 0.5 ± 0.2 303 0.9 ± 0.2 308 1.5 ± 0.2 313 Ea (kJ.mol -1 ) 39 ∆H ‡ (kJ.mol -1 ) ∆S ‡ (J.mol -1 .K -1 ) 90.3 ± 0.1 87.8 ± 0.1 -11 ± 4 105.5 ± 0.1 102.9 ± 0.1 31 ± 4 The kapp values were fit to the Arrhenius plot, kapp = Aexp(-Ea/RT), and the Ea values for the two processes were calculated (Table 2.7). The activation parameters, ∆H ‡ and ∆S ‡ , were determined from the Eyring plot. The differences in the activation energy of the two processes were attributed to the more stable bond between the dbm ligand and the niobium atom in complex 2, through a delocalized π system due to the phenyl rings of the ligand, than in complex 1 with the dpm ligand In 2003 Aresta et al. 86 published an article on CO2 insertion into Nb-OR bonds of various Nb(OR)5 (R = Me, Et, allyl) complexes. Their study also included the absorption kinetics of CO2 insertion into Nb(OEt)5. As mentioned before, the niobium penta-alkoxo complexes are dimeric in the solid state and in solution. They conducted a 1 H NMR investigation and found that [Nb(OEt)5]2 undergoes a dissociation equilibrium in solution. The dissociation equilibrium (Keq) was calculated as 2.07 x 10 -4 . Figure 2.18: Reaction of niobium penta ethoxide with CO2. 86 M. Aresta, A. Dibenedetto, C. Pastore, Inorg. Chem., 42, 10, 3256, 2003.
Chapter 2 The hemicarbonate formed in the CO2 insertion reaction can function as a η 1 -mono- dentate ligand (a), a η 2 -bidentate ligand (b) or a η 2 -µ 2 ligand (c), bridging two metal centers as displayed in Figure 2.19: (a) (b) (c) Figure 2.19: Mode of bonding of hemicarbonate. The kinetics of absorption of CO2 by [Nb(OEt)5]2 was done in EtOH and benzene that had been saturated with CO2 in an absorption cell connected to a gas buret. The gas adsorption was monitored for 70 h at 298 K. The reaction was stopped when the CO2 uptake reached 0.9 mol/(mol niobium) and the product was isolated and confirmed to be [Nb(OEt)4(OC(O)OEt)]. Figure 2.20: Kinetics of absorption of CO2 by [Nb(OEt)5]2 in ethanol (blue) and benzene (red). 87 40
- Page 1 and 2: A solution and solid state study of
- Page 3 and 4: Table of contents Abbreviations and
- Page 5 and 6: 3.4 Infrared Spectroscopy (IR) ....
- Page 7 and 8: Abbreviations and Symbols Abbreviat
- Page 9 and 10: Abstract 93 Nb NMR was successfully
- Page 11 and 12: Opsomming 93 Nb KMR is met sukses g
- Page 13 and 14: Chapter 1 metals. 3 Due to Wollasto
- Page 15 and 16: Synopsis... 2. Literature Review of
- Page 17 and 18: Chapter 2 Niobium resembles tantalu
- Page 19 and 20: 2.1.2 Uses Chapter 2 Niobium has a
- Page 21 and 22: 2.2 Separation of Nb and Ta 2.2.1 M
- Page 23 and 24: Chapter 2 Buachuang et al. 16 repor
- Page 25 and 26: Chapter 2 Niobium oxide surfaces ex
- Page 27 and 28: 2.4.6 Water absorption Chapter 2 Th
- Page 29 and 30: Chapter 2 been reported in literatu
- Page 31 and 32: Chapter 2 containing niobium as the
- Page 33 and 34: Chapter 2 conclusions, with regard
- Page 35 and 36: Chapter 2 Figure 2.5: Structure of
- Page 37 and 38: 2.6.3.3 [NbCl3O(ttbd) - ] Chapter 2
- Page 39 and 40: Chapter 2 (a) (b) Figure 2.10: Stru
- Page 41 and 42: 2.7 Alkoxides Chapter 2 Specific kn
- Page 43 and 44: Chapter 2 Reactions of dialkylamid
- Page 45 and 46: Chapter 2 other NbCl5-x(OMe)x produ
- Page 47 and 48: Chapter 2 In 1991 Lee et al. 83 pub
- Page 49: EtO EtO EtO EtO Cl Nb Cl Cl Nb Cl R
- Page 53 and 54: Synopsis... 3. Synthesis and Charac
- Page 55 and 56: Chapter 3 with γ = magnetogyric ra
- Page 57 and 58: Chapter 3 electromagnetic spectrum
- Page 59 and 60: 3.5.1 Bragg’s law Chapter 3 Bragg
- Page 61 and 62: 3.5.3 ‘Phase problem’ Chapter 3
- Page 63 and 64: Chapter 3 A = ∑ ε cl (3.14) In
- Page 65 and 66: Chapter 3 t1/2 = 54 = . 3.7 Sy
- Page 67 and 68: 3.7.2.4 Synthesis of [NbCl4(acac)]:
- Page 69 and 70: 4. Crystallographic Synopsis... Cha
- Page 71 and 72: Chapter 4 packages 2 respectively.
- Page 73 and 74: Chapter 4 4.3 Crystal Structure of
- Page 75 and 76: Chapter 4 longer bonds (C1-C2, C2-C
- Page 77 and 78: Chapter 4 Figure 4.5: Packing of [N
- Page 79 and 80: Chapter 4 Figure 4.7: Molecular str
- Page 81 and 82: Chapter 4 Table 4.5: Selected bond
- Page 83 and 84: Chapter 4 Figure 4.11: Molecular st
- Page 85 and 86: Chapter 4 Figure 4.12: The phacac p
- Page 87 and 88: Chapter 4 Table 4.9: Hydrogen bonds
- Page 89 and 90: Chapter 4 According to our knowledg
- Page 91 and 92: 5.2 Experimental procedures 5.2.1 K
- Page 93 and 94: Chapter 5 corresponds to one specie
- Page 95 and 96: 5.3 Results and Discussion 5.3.1 Pr
- Page 97 and 98: Chapter 5 Figure 5.5: Typical UV/Vi
- Page 99 and 100: 5.3.3 Derivation of the rate law Ch
Chapter 2<br />
Table 2.7: Kinetic <strong>and</strong> thermodynamic parameters on the displacement reactions <strong>of</strong> complex<br />
1 <strong>and</strong> 2 with Hacac. 86<br />
Experiment kapp x 10 3<br />
1<br />
2<br />
(s -1 )<br />
T (K)<br />
0.3 ± 0.2 293<br />
0.6 ± 0.2 298<br />
1.2 ± 0.2 303<br />
2.1 ± 0.2 308<br />
3.1 ± 0.2 313<br />
0.1 ± 0.2 293<br />
0.2 ± 0.2 298<br />
0.5 ± 0.2 303<br />
0.9 ± 0.2 308<br />
1.5 ± 0.2 313<br />
Ea<br />
(kJ.mol -1 )<br />
39<br />
∆H ‡<br />
(kJ.mol -1 )<br />
∆S ‡<br />
(J.mol -1 .K -1 )<br />
90.3 ± 0.1 87.8 ± 0.1 -11 ± 4<br />
105.5 ± 0.1 102.9 ± 0.1 31 ± 4<br />
The kapp values were fit to the Arrhenius plot, kapp = Aexp(-Ea/RT), <strong>and</strong> the Ea values<br />
for the two processes were calculated (Table 2.7). The activation parameters, ∆H ‡<br />
<strong>and</strong> ∆S ‡ , were determined from the Eyring plot. The differences in the activation<br />
energy <strong>of</strong> the two processes were attributed to the more stable bond between the<br />
dbm lig<strong>and</strong> <strong>and</strong> the <strong>niobium</strong> atom in complex 2, through a delocalized π system due<br />
to the phenyl rings <strong>of</strong> the lig<strong>and</strong>, than in complex 1 with the dpm lig<strong>and</strong><br />
In 2003 Aresta et al. 86 published an article on CO2 insertion into Nb-OR bonds <strong>of</strong><br />
various Nb(OR)5 (R = Me, Et, allyl) <strong>complexes</strong>. Their <strong>study</strong> also included the<br />
absorption kinetics <strong>of</strong> CO2 insertion into Nb(OEt)5. As mentioned before, the <strong>niobium</strong><br />
penta-alkoxo <strong>complexes</strong> are dimeric in the <strong>solid</strong> <strong>state</strong> <strong>and</strong> in <strong>solution</strong>. They<br />
conducted a 1 H NMR investigation <strong>and</strong> found that [Nb(OEt)5]2 undergoes a<br />
dissociation equilibrium in <strong>solution</strong>. The dissociation equilibrium (Keq) was calculated<br />
as 2.07 x 10 -4 .<br />
Figure 2.18: Reaction <strong>of</strong> <strong>niobium</strong> penta ethoxide with CO2.<br />
86 M. Aresta, A. Dibenedetto, C. Pastore, Inorg. Chem., 42, 10, 3256, 2003.