A solution and solid state study of niobium complexes University of ...
A solution and solid state study of niobium complexes University of ... A solution and solid state study of niobium complexes University of ...
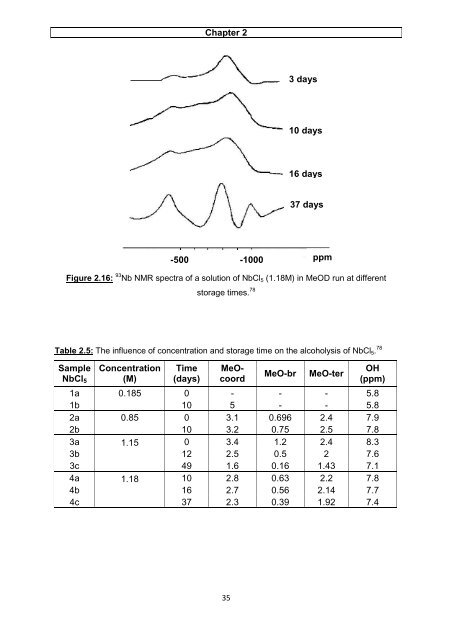
Chapter 2 Figure 2.16: 93 Nb NMR spectra of a solution of NbCl5 (1.18M) in MeOD run at different storage times. 78 Table 2.5: The influence of concentration and storage time on the alcoholysis of NbCl5. 78 Sample NbCl5 Concentration (M) Time (days) MeOcoord 35 3 days 10 days 16 days 37 days -500 -1000 ppm MeO-br MeO-ter OH (ppm) 1a 0.185 0 - - - 5.8 1b 10 5 - - 5.8 2a 0.85 0 3.1 0.696 2.4 7.9 2b 10 3.2 0.75 2.5 7.8 3a 1.15 0 3.4 1.2 2.4 8.3 3b 12 2.5 0.5 2 7.6 3c 49 1.6 0.16 1.43 7.1 4a 1.18 10 2.8 0.63 2.2 7.8 4b 16 2.7 0.56 2.14 7.7 4c 37 2.3 0.39 1.92 7.4
Chapter 2 In 1991 Lee et al. 83 published a paper where they used 93 Nb- and 1 H NMR spectroscopy to investigate the different substitution products of [NbCl5-x(OMe)x] that forms in the stepwise substitution of NbCl5 by MeOH in non co-ordinating solvents. Their results confirmed Schönherr’s et al. 84 results that only [NbCl2(OMe)3] is formed on adding an excess MeOH to NbCl5 in non-polar solvents. To validate their results they isolated and characterized [NbCl4(OMe)], [NbCl2(OMe)3] and [Nb(OMe)5] as reference samples. They did not isolate [NbCl3(OMe)2] as it readily disproportionates to form [NbCl2(OMe)3] and [NbCl4(OMe)]. This is due to the lower solubility of [NbCl4(OMe)] and the stability of [NbCl2(OMe)3]. The coordination of NbCl5 with MeOH in different aromatic solvents was followed by 93 Nb NMR and compared to the relative reference peaks. The results are presented in Table 2.6. Table 2.6: Assignment of 93 Nb NMR signals to NbCl5-x(OMe)x species. 84 Solvent x = 0 x = 1 x = 3 x = 4 x = 5 Benzene 2.6 -497 -810 -1010 -1160 Toluene 2 -495 -810 - -1150 Methanol -0.5 -480 -820 -1015 -1155 Ethanol - -470 -830 -1020 -1180 Isopropanol - -470 -830 -1025 -1135 Acetonitrile - -495 / -560 -850 - -1160 2.8 Kinetic Studies of Niobium Complexes Antiñolo et al. 85 published a paper in 2000 in which they studied the reaction of niobium alkoxo complexes with different β-diketones. The β-diketones of choice was tBuCOCH2COtBu (Hdpm), PhCOCH2COPh (Hdbm) and acacH. They followed the kinetics of the displacement reaction of [NbCl2(OEt)2(dpm)] (complex 1) and [NbCl2(OEt)2(dbm)] (complex 2) with acacH to give [NbCl2(OEt)2(acac)] as seen in Figure 2.17. 83 G. R. Lee, J. A. Crayston, Dalton Trans., 3073, 1991. 84 M. Schönherr, L. Kolditz, Z. Chem., 10, 72, 1970. 85 A. Antiñolo, F. Carrillo-Hermosilla, J. Fernández-Baeza, S. Garcia-Yuste, A. Otera, E. Palomares, A. M. Rodriguez, L. F. Sánchez-Barba, J. Organomet. Chem., 603, 194, 2000. 36
- Page 1 and 2: A solution and solid state study of
- Page 3 and 4: Table of contents Abbreviations and
- Page 5 and 6: 3.4 Infrared Spectroscopy (IR) ....
- Page 7 and 8: Abbreviations and Symbols Abbreviat
- Page 9 and 10: Abstract 93 Nb NMR was successfully
- Page 11 and 12: Opsomming 93 Nb KMR is met sukses g
- Page 13 and 14: Chapter 1 metals. 3 Due to Wollasto
- Page 15 and 16: Synopsis... 2. Literature Review of
- Page 17 and 18: Chapter 2 Niobium resembles tantalu
- Page 19 and 20: 2.1.2 Uses Chapter 2 Niobium has a
- Page 21 and 22: 2.2 Separation of Nb and Ta 2.2.1 M
- Page 23 and 24: Chapter 2 Buachuang et al. 16 repor
- Page 25 and 26: Chapter 2 Niobium oxide surfaces ex
- Page 27 and 28: 2.4.6 Water absorption Chapter 2 Th
- Page 29 and 30: Chapter 2 been reported in literatu
- Page 31 and 32: Chapter 2 containing niobium as the
- Page 33 and 34: Chapter 2 conclusions, with regard
- Page 35 and 36: Chapter 2 Figure 2.5: Structure of
- Page 37 and 38: 2.6.3.3 [NbCl3O(ttbd) - ] Chapter 2
- Page 39 and 40: Chapter 2 (a) (b) Figure 2.10: Stru
- Page 41 and 42: 2.7 Alkoxides Chapter 2 Specific kn
- Page 43 and 44: Chapter 2 Reactions of dialkylamid
- Page 45: Chapter 2 other NbCl5-x(OMe)x produ
- Page 49 and 50: EtO EtO EtO EtO Cl Nb Cl Cl Nb Cl R
- Page 51 and 52: Chapter 2 The hemicarbonate formed
- Page 53 and 54: Synopsis... 3. Synthesis and Charac
- Page 55 and 56: Chapter 3 with γ = magnetogyric ra
- Page 57 and 58: Chapter 3 electromagnetic spectrum
- Page 59 and 60: 3.5.1 Bragg’s law Chapter 3 Bragg
- Page 61 and 62: 3.5.3 ‘Phase problem’ Chapter 3
- Page 63 and 64: Chapter 3 A = ∑ ε cl (3.14) In
- Page 65 and 66: Chapter 3 t1/2 = 54 = . 3.7 Sy
- Page 67 and 68: 3.7.2.4 Synthesis of [NbCl4(acac)]:
- Page 69 and 70: 4. Crystallographic Synopsis... Cha
- Page 71 and 72: Chapter 4 packages 2 respectively.
- Page 73 and 74: Chapter 4 4.3 Crystal Structure of
- Page 75 and 76: Chapter 4 longer bonds (C1-C2, C2-C
- Page 77 and 78: Chapter 4 Figure 4.5: Packing of [N
- Page 79 and 80: Chapter 4 Figure 4.7: Molecular str
- Page 81 and 82: Chapter 4 Table 4.5: Selected bond
- Page 83 and 84: Chapter 4 Figure 4.11: Molecular st
- Page 85 and 86: Chapter 4 Figure 4.12: The phacac p
- Page 87 and 88: Chapter 4 Table 4.9: Hydrogen bonds
- Page 89 and 90: Chapter 4 According to our knowledg
- Page 91 and 92: 5.2 Experimental procedures 5.2.1 K
- Page 93 and 94: Chapter 5 corresponds to one specie
- Page 95 and 96: 5.3 Results and Discussion 5.3.1 Pr
Chapter 2<br />
Figure 2.16: 93 Nb NMR spectra <strong>of</strong> a <strong>solution</strong> <strong>of</strong> NbCl5 (1.18M) in MeOD run at different<br />
storage times. 78<br />
Table 2.5: The influence <strong>of</strong> concentration <strong>and</strong> storage time on the alcoholysis <strong>of</strong> NbCl5. 78<br />
Sample<br />
NbCl5<br />
Concentration<br />
(M)<br />
Time<br />
(days)<br />
MeOcoord<br />
35<br />
3 days<br />
10 days<br />
16 days<br />
37 days<br />
-500 -1000 ppm<br />
MeO-br MeO-ter<br />
OH<br />
(ppm)<br />
1a 0.185 0 - - - 5.8<br />
1b 10 5 - - 5.8<br />
2a 0.85 0 3.1 0.696 2.4 7.9<br />
2b 10 3.2 0.75 2.5 7.8<br />
3a 1.15 0 3.4 1.2 2.4 8.3<br />
3b 12 2.5 0.5 2 7.6<br />
3c 49 1.6 0.16 1.43 7.1<br />
4a 1.18 10 2.8 0.63 2.2 7.8<br />
4b 16 2.7 0.56 2.14 7.7<br />
4c 37 2.3 0.39 1.92 7.4