A solution and solid state study of niobium complexes University of ...
A solution and solid state study of niobium complexes University of ...
A solution and solid state study of niobium complexes University of ...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
Chapter 2<br />
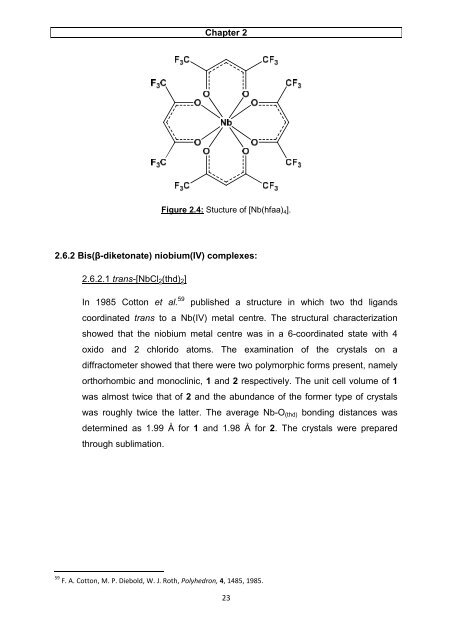
Figure 2.4: Stucture <strong>of</strong> [Nb(hfaa)4].<br />
2.6.2 Bis(β-diketonate) <strong>niobium</strong>(IV) <strong>complexes</strong>:<br />
2.6.2.1 trans-[NbCl2(thd)2]<br />
In 1985 Cotton et al. 59 published a structure in which two thd lig<strong>and</strong>s<br />
coordinated trans to a Nb(IV) metal centre. The structural characterization<br />
showed that the <strong>niobium</strong> metal centre was in a 6-coordinated <strong>state</strong> with 4<br />
oxido <strong>and</strong> 2 chlorido atoms. The examination <strong>of</strong> the crystals on a<br />
diffractometer showed that there were two polymorphic forms present, namely<br />
orthorhombic <strong>and</strong> monoclinic, 1 <strong>and</strong> 2 respectively. The unit cell volume <strong>of</strong> 1<br />
was almost twice that <strong>of</strong> 2 <strong>and</strong> the abundance <strong>of</strong> the former type <strong>of</strong> crystals<br />
was roughly twice the latter. The average Nb-O(thd) bonding distances was<br />
determined as 1.99 Å for 1 <strong>and</strong> 1.98 Å for 2. The crystals were prepared<br />
through sublimation.<br />
59 F. A. Cotton, M. P. Diebold, W. J. Roth, Polyhedron, 4, 1485, 1985.<br />
23