Tuning Reactivity of Platinum(II) Complexes
Tuning Reactivity of Platinum(II) Complexes Tuning Reactivity of Platinum(II) Complexes
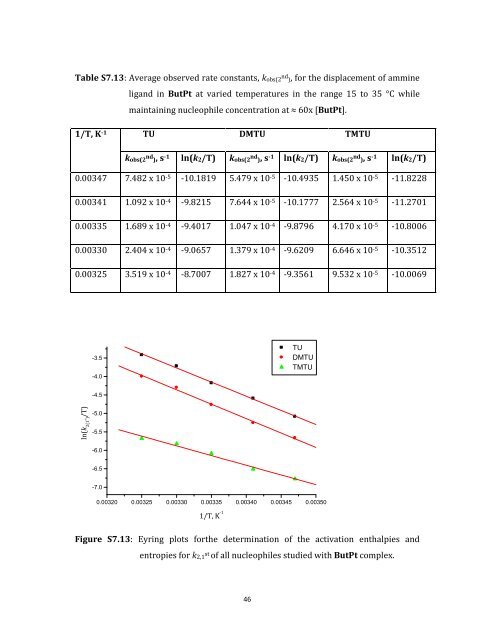
Table S7.13: Average observed rate constants, kobs(2 nd ), for the displacement of ammine ligand in ButPt at varied temperatures in the range 15 to 35 °C while maintaining nucleophile concentration at ≈ 60x [ButPt]. 1/T, K -1 TU DMTU TMTU kobs(2 nd ), s -1 ln(k2/T) kobs(2 nd ), s -1 ln(k2/T) kobs(2 nd ), s -1 ln(k2/T) 0.00347 7.482 x 10 -5 -10.1819 5.479 x 10 -5 -10.4935 1.450 x 10 -5 -11.8228 0.00341 1.092 x 10 -4 -9.8215 7.644 x 10 -5 -10.1777 2.564 x 10 -5 -11.2701 0.00335 1.689 x 10 -4 -9.4017 1.047 x 10 -4 -9.8796 4.170 x 10 -5 -10.8006 0.00330 2.404 x 10 -4 -9.0657 1.379 x 10 -4 -9.6209 6.646 x 10 -5 -10.3512 0.00325 3.519 x 10 -4 -8.7007 1.827 x 10 -4 -9.3561 9.532 x 10 -5 -10.0069 ln(k 2(1 st ) /T) -3.5 -4.0 -4.5 -5.0 -5.5 -6.0 -6.5 -7.0 0.00320 0.00325 0.00330 0.00335 0.00340 0.00345 0.00350 1/T, K -1 46 TU DMTU TMTU Figure S7.13: Eyring plots forthe determination of the activation enthalpies and entropies for k2,1 st of all nucleophiles studied with ButPt complex.
ln(k nd 2(2 ) /T) -9 -10 -11 -12 0.00320 0.00325 0.00330 0.00335 0.00340 0.00345 0.00350 1/T, K -1 47 TU DMTU TMTU FigureS7.14: Eyring plots forthe determination of the activation enthalpies and Absorbance 2. 0 1. 5 1. 0 0. 5 0. 0 entropies for k2,2 nd of all nucleophiles studied with ButPt complex. Absorbance 0 .01 8 0 .01 7 0 .01 6 0 .01 5 0 .01 4 2 3 4 5 6 7 2 00 225 2 5 0 2 75 300 Wavelength (nm) pH λ = 238 nm Figure S7.15: UV-Vis spectra recorded for the ButPt complex in the pH range 2-10 at 25 °C. Inset: Plot of absorbance versus pH at 238 nm.
- Page 352 and 353: Absorbance 1.6 1.4 1.2 1.0 0.8 0.6
- Page 354 and 355: SH N SH + Mechanism Br N + CO 3 2-
- Page 356 and 357: Figure 7.5: 195Pt NMR spectra of mi
- Page 358 and 359: linker remained coordinated to the
- Page 360 and 361: complexes, have a lower charge and
- Page 362 and 363: ange 326-400 cm -1 (weak) for Pt-Cl
- Page 364 and 365: ButPt, HexPt, OctPt and DecPt, were
- Page 366 and 367: 7.3 Results 7.3.1 DFT Calculations
- Page 368 and 369: Structure HOMO LUMO EnPt (C2h) Prop
- Page 370 and 371: Absorbance Table 7.2: Summary of pK
- Page 372 and 373: coordination to the soft Pt(II) cen
- Page 374 and 375: observed at -2962.4 and -3024.1 ppm
- Page 376 and 377: H 3N NH 3 Pt NH 2 OH 2 n NH 3 NH 2
- Page 378 and 379: k obs2 , in s -1 -3 TU 1.2x10 DMTU
- Page 380 and 381: 7.3.4 Activation Parameters The tem
- Page 382 and 383: density is located on the metal cen
- Page 384 and 385: acetylmethionine, which reported th
- Page 386 and 387: References 1 (a) B. Rosenberg, L. V
- Page 388 and 389: 32 N. Summa, J. Maigut, R. Puchta a
- Page 390 and 391: Appendix 7 Table S7.1: Summary of s
- Page 392 and 393: k obs(2 nd ) , in s -1 0.00020 0.00
- Page 394 and 395: ln(k 2(2 nd ) /T) -8.5 -9.0 -9.5 -1
- Page 396 and 397: k obs(1 st ) , in s -1 0.06 TU DMTU
- Page 398 and 399: ln(k 2(1 st ) /T) -3.2 TU DMTU TMTU
- Page 400 and 401: Table S7.11: Summary of kobs(2 nd )
- Page 404 and 405: %T 22.0 20 18 16 14 12 10 8 6 4 2 0
- Page 406 and 407: k st obs(1 ) , in s-1 0.10 0.08 0.0
- Page 408 and 409: ln(k st 2(1 ) /T) -4.0 TU DMTU TMTU
- Page 410 and 411: Figure S7.23: Mass spectra for HexP
- Page 412 and 413: Table S21: Average observed rate co
- Page 414 and 415: Table S7.23: Summary of kobs(2 nd )
- Page 416 and 417: Table S7.25: Average observed rate
- Page 418 and 419: 90.0 80 70 60 50 40 30 20 %T 10 0 -
- Page 420 and 421: Figure S7.37: Mass spectrum for Hex
- Page 422 and 423: Chapter 8 Tuning Reactivity of plat
- Page 424 and 425: dinuclear Pt(II) complexes to relea
- Page 426 and 427: finally Pt2. The order of reactivit
- Page 428: • prolonged survival in the cell
Table S7.13: Average observed rate constants, kobs(2 nd ), for the displacement <strong>of</strong> ammine<br />
ligand in ButPt at varied temperatures in the range 15 to 35 °C while<br />
maintaining nucleophile concentration at ≈ 60x [ButPt].<br />
1/T, K -1 TU DMTU TMTU<br />
kobs(2 nd ), s -1 ln(k2/T) kobs(2 nd ), s -1 ln(k2/T) kobs(2 nd ), s -1 ln(k2/T)<br />
0.00347 7.482 x 10 -5 -10.1819 5.479 x 10 -5 -10.4935 1.450 x 10 -5 -11.8228<br />
0.00341 1.092 x 10 -4 -9.8215 7.644 x 10 -5 -10.1777 2.564 x 10 -5 -11.2701<br />
0.00335 1.689 x 10 -4 -9.4017 1.047 x 10 -4 -9.8796 4.170 x 10 -5 -10.8006<br />
0.00330 2.404 x 10 -4 -9.0657 1.379 x 10 -4 -9.6209 6.646 x 10 -5 -10.3512<br />
0.00325 3.519 x 10 -4 -8.7007 1.827 x 10 -4 -9.3561 9.532 x 10 -5 -10.0069<br />
ln(k 2(1 st ) /T)<br />
-3.5<br />
-4.0<br />
-4.5<br />
-5.0<br />
-5.5<br />
-6.0<br />
-6.5<br />
-7.0<br />
0.00320 0.00325 0.00330 0.00335 0.00340 0.00345 0.00350<br />
1/T, K -1<br />
46<br />
TU<br />
DMTU<br />
TMTU<br />
Figure S7.13: Eyring plots forthe determination <strong>of</strong> the activation enthalpies and<br />
entropies for k2,1 st <strong>of</strong> all nucleophiles studied with ButPt complex.