Processing kodak motion picture films, module 3 analytical procedures
Processing kodak motion picture films, module 3 analytical procedures Processing kodak motion picture films, module 3 analytical procedures
REPRODUCIBILITY Customer Standard Deviation, 1sc & 95 Percent Confidence Estimate (not including bias) The Reproducibility or customer standard deviation (1sc ) is an estimate of the variability a customer could expect when submitting a sample to any Photoprocessing Quality Services laboratory, where any trained analyst could test the sample using any instrument on any day. The 95 percent confidence estimate (calculated using the customer standard deviation) around a single test result will include the mean value 95 percent of the time. Four analysts analyzed four Accelerator tank samples, on two different days. Duplicate analyses were performed on each sample, on each of the two days. These samples were: 1. A “fresh” Accelerator tank solution prepared with all components at their respective “working tank” aim concentrations. 2. A “seasoned” Accelerator tank solution analyzed as received as 3.55 g/L PBA-1. 3. The same “seasoned” solution, as in number 2, above, analyzed in the same manner, after making a standard addition of 1.2327 g/L PBA-1. Sample “Fresh” (prepared at 3.31 g/L) “Seasoned” As Received “Seasoned” plus Standard Addition Mean (g/L PBA-1) N PBA-1 Reproducibility Standard Deviation, 1s c (g/L PBA-1) 95 Percent Confidence Estimate (g/L PBA-1) 3.31 16 0.104 ± 0.22 3.55 16 0.022 ± 0.05 4.81 16 0.040 ± 0.09 Bias Bias is a statistically significant deviation of the mean from the known mix level at a 95 percent confidence level. It is determined for fresh samples only. Bias was not determined for this sample because the component concentration level was not determined independently of the test method. No statistically significant bias was found at the 95 percent confidence level. Recovery Recovery is used for seasoned samples, since the component concentration level was not determined independently of the test method. It is defined as the calculated mean for the seasoned sample with a standard addition of the component minus the mean for the seasoned sample, divided by the actual amount of the standard addition. It is expressed as a percentage. Statistically, the recovery of 102.21 percent was significantly different from 100 percent at the 95 percent confidence level, however it was judged not to be practically significant. APPARATUS Metrohm E536 Autotitrator or equivalent Orion Silver-Silver Sulfide Electrode, Catalog No. 941600 or equivalent (Note: DO NOT use a silver billet or bar electrode as prepared in SLM-1295) Orion Double Junction Reference Electrode, Catalog No. 90-02-00 or equivalent Pipet (50.0 mL) Tip-up pipet (10 mL) Graduated Cylinder (250 mL) All volumetric glassware should meet all Class A specifications, as defined by American Society for Testing and Materials (ASTM) Standards E 287, E288, and E969, unless otherwise stated. REAGENTS All reagents should be ACS Reagent Grade unless otherwise specified. 10 N Sodium Hydroxide, NaOH 0.0500 N Silver Nitrate, AgNO3 (standardized to four decimal places) Water, Type I Reagent - This method was developed using reagent water equivalent to or purer than Type I grade, as defined in ASTM Standard D 1193. Other grades of water, e.g., reverse osmosis (RO), demineralized, or distilled water, may give equivalent results, but the effects of water quality on method performance have not been studied. 2 Processing KODAK Motion Picture Films, Module 3, Analytical Procedures H24.03
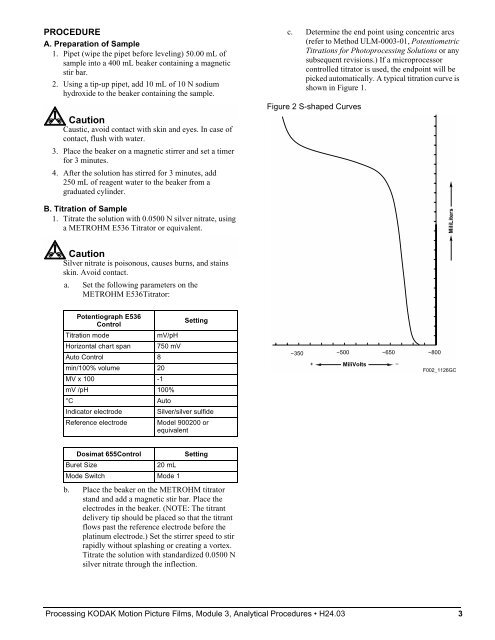
PROCEDURE A. Preparation of Sample 1. Pipet (wipe the pipet before leveling) 50.00 mL of sample into a 400 mL beaker containing a magnetic stir bar. 2. Using a tip-up pipet, add 10 mL of 10 N sodium hydroxide to the beaker containing the sample. Caution Caustic, avoid contact with skin and eyes. In case of contact, flush with water. 3. Place the beaker on a magnetic stirrer and set a timer for 3 minutes. 4. After the solution has stirred for 3 minutes, add 250 mL of reagent water to the beaker from a graduated cylinder. B. Titration of Sample 1. Titrate the solution with 0.0500 N silver nitrate, using a METROHM E536 Titrator or equivalent. Caution Silver nitrate is poisonous, causes burns, and stains skin. Avoid contact. a. Set the following parameters on the METROHM E536Titrator: Potentiograph E536 Control Setting Titration mode mV/pH Horizontal chart span 750 mV Auto Control 8 min/100% volume 20 MV x 100 -1 mV /pH 100% °C Auto Indicator electrode Silver/silver sulfide Reference electrode Model 900200 or equivalent Dosimat 655Control Setting Buret Size 20 mL Mode Switch Mode 1 b. Place the beaker on the METROHM titrator stand and add a magnetic stir bar. Place the electrodes in the beaker. (NOTE: The titrant delivery tip should be placed so that the titrant flows past the reference electrode before the platinum electrode.) Set the stirrer speed to stir rapidly without splashing or creating a vortex. Titrate the solution with standardized 0.0500 N silver nitrate through the inflection. c. Determine the end point using concentric arcs (refer to Method ULM-0003-01, Potentiometric Titrations for Photoprocessing Solutions or any subsequent revisions.) If a microprocessor controlled titrator is used, the endpoint will be picked automatically. A typical titration curve is shown in Figure 1. Figure 2 S-shaped Curves Processing KODAK Motion Picture Films, Module 3, Analytical Procedures H24.03 3 −350 −500 −650 + MiliVolts − −800 MiliLiters F002_1126GC
- Page 123 and 124: Iodometric Determination of Sulfite
- Page 125 and 126: Potentiometric Determination of Tot
- Page 127 and 128: Automated Titration An example of a
- Page 129 and 130: Buffering Capacity Determination of
- Page 131 and 132: Buffering Capacity Determination of
- Page 133 and 134: Titrimetric Determination of EASTMA
- Page 135 and 136: VISUAL TITRATION STATISTICS Repeata
- Page 137 and 138: Titration of the Developing Agent w
- Page 139 and 140: Cerimetric Determination of CD-2 Co
- Page 141 and 142: Cerimetric Determination of KODAK C
- Page 143 and 144: Back-Extraction of CD-2 1. Add 50 m
- Page 145 and 146: Potentiometric Determination of Fer
- Page 147 and 148: Recovery Recovery is used instead o
- Page 149 and 150: CALCULATIONS For Na3Fe(CN) 6 g/L Na
- Page 151 and 152: Potentiometric Determination of Fer
- Page 153 and 154: Bias Bias is a statistically signif
- Page 155 and 156: Spectrophotometric Determination of
- Page 157 and 158: Hydroquinone in Sound Track Develop
- Page 159 and 160: Titrimetric Determination of Hypo I
- Page 161 and 162: Recovery Recovery is used instead o
- Page 163 and 164: Recovery Recovery is used instead o
- Page 165 and 166: B. Thiosulfate Determination 1. Sam
- Page 167 and 168: Examples: Titration mL 0.1 N Na 2S
- Page 169 and 170: Potentiometric Determination of Pot
- Page 171 and 172: APPARATUS All volumetric glassware
- Page 173: Potentiometric Determination of Kod
- Page 177 and 178: Titrimetric Determination of Persul
- Page 179 and 180: APPARATUS Conical Flask with stoppe
- Page 181 and 182: Determination of the pH of the East
- Page 183 and 184: Determination of the pH of Processe
- Page 185 and 186: Potentiometric Determination of Sil
- Page 187 and 188: APPARATUS METROHM 536 Titrator or e
- Page 189 and 190: Determination of Sodium Metabisulfi
- Page 191 and 192: II. Visual Endpoint Titrations A. R
- Page 193 and 194: APPARATUS METROHM 536 Titrator or e
- Page 195 and 196: Viscosity Determination of Sound-Tr
- Page 197 and 198: Titrimetric Determination Of Benzyl
- Page 199 and 200: Potentiometric Determination of Bro
- Page 201 and 202: PROCEDURE B For Seasoned Tank Note:
- Page 203 and 204: Potentiometric Determination of Bro
- Page 205 and 206: CALCULATIONS mL AgNO 3 that would b
- Page 207 and 208: Titrimetric Determination of Buffer
- Page 209 and 210: Potentiometric Determination of Kod
- Page 211 and 212: Spectrophotometric Determination of
- Page 213 and 214: Titrimetric Determination of Citraz
- Page 215 and 216: Potentiometric Determination of Eth
- Page 217 and 218: Titrimetric Determination of Ferric
- Page 219 and 220: Iodometric Determination of Ferricy
- Page 221 and 222: Potentiometric Determination of Fer
- Page 223 and 224: Iodometric Determination of Formali
PROCEDURE<br />
A. Preparation of Sample<br />
1. Pipet (wipe the pipet before leveling) 50.00 mL of<br />
sample into a 400 mL beaker containing a magnetic<br />
stir bar.<br />
2. Using a tip-up pipet, add 10 mL of 10 N sodium<br />
hydroxide to the beaker containing the sample.<br />
Caution<br />
Caustic, avoid contact with skin and eyes. In case of<br />
contact, flush with water.<br />
3. Place the beaker on a magnetic stirrer and set a timer<br />
for 3 minutes.<br />
4. After the solution has stirred for 3 minutes, add<br />
250 mL of reagent water to the beaker from a<br />
graduated cylinder.<br />
B. Titration of Sample<br />
1. Titrate the solution with 0.0500 N silver nitrate, using<br />
a METROHM E536 Titrator or equivalent.<br />
Caution<br />
Silver nitrate is poisonous, causes burns, and stains<br />
skin. Avoid contact.<br />
a. Set the following parameters on the<br />
METROHM E536Titrator:<br />
Potentiograph E536<br />
Control<br />
Setting<br />
Titration mode mV/pH<br />
Horizontal chart span 750 mV<br />
Auto Control 8<br />
min/100% volume 20<br />
MV x 100 -1<br />
mV /pH 100%<br />
°C Auto<br />
Indicator electrode Silver/silver sulfide<br />
Reference electrode Model 900200 or<br />
equivalent<br />
Dosimat 655Control Setting<br />
Buret Size 20 mL<br />
Mode Switch Mode 1<br />
b. Place the beaker on the METROHM titrator<br />
stand and add a magnetic stir bar. Place the<br />
electrodes in the beaker. (NOTE: The titrant<br />
delivery tip should be placed so that the titrant<br />
flows past the reference electrode before the<br />
platinum electrode.) Set the stirrer speed to stir<br />
rapidly without splashing or creating a vortex.<br />
Titrate the solution with standardized 0.0500 N<br />
silver nitrate through the inflection.<br />
c. Determine the end point using concentric arcs<br />
(refer to Method ULM-0003-01, Potentiometric<br />
Titrations for Photoprocessing Solutions or any<br />
subsequent revisions.) If a microprocessor<br />
controlled titrator is used, the endpoint will be<br />
picked automatically. A typical titration curve is<br />
shown in Figure 1.<br />
Figure 2 S-shaped Curves<br />
<strong>Processing</strong> KODAK Motion Picture Films, Module 3, Analytical Procedures H24.03 3<br />
−350<br />
−500<br />
−650<br />
+ MiliVolts<br />
−<br />
−800<br />
MiliLiters<br />
F002_1126GC