Lunesta Letter - Haymarket Media Group
Lunesta Letter - Haymarket Media Group
Lunesta Letter - Haymarket Media Group
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
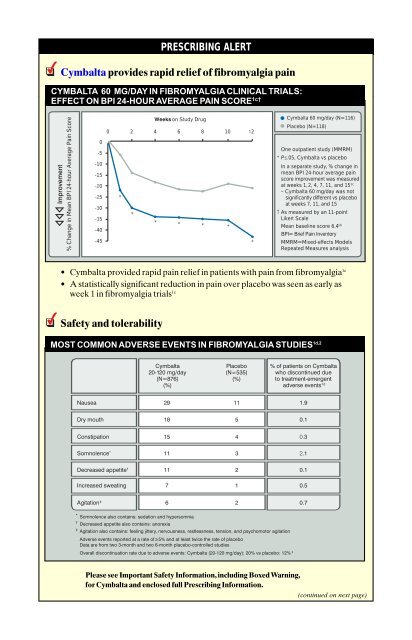
Cymbalta provides rapid relief of fibromyalgia pain<br />
• Cymbalta provided rapid pain relief in patients with pain from fibromyalgia 1c<br />
• A statistically significant reduction in pain over placebo was seen as early as<br />
week 1 in fibromyalgia trials 1c<br />
Safety and tolerability<br />
Nausea<br />
Dry mouth<br />
Constipation<br />
Somnolence *<br />
Decreased appetite †<br />
Increased sweating<br />
Agitation ‡<br />
PRESCRIBING ALERT<br />
CYMBALTA 60 MG/DAY IN FIBROMYALGIA CLINICAL TRIALS:<br />
EFFECT ON BPI 24-HOUR AVERAGE PAIN SCORE 1c†<br />
Improvement<br />
% Change in Mean BPI 24-hour Average Pain Score<br />
0<br />
-5<br />
-10<br />
-15<br />
-20<br />
-25<br />
-30<br />
-35<br />
-40<br />
-45<br />
Weeks on Study Drug<br />
0 2 4 6 8 10 12<br />
*<br />
*<br />
*<br />
MOST COMMON ADVERSE EVENTS IN FIBROMYALGIA STUDIES 1d,2<br />
Cymbalta<br />
20-120 mg/day<br />
(N=876)<br />
(%)<br />
29<br />
18<br />
15<br />
11<br />
11<br />
7<br />
6<br />
*<br />
Placebo<br />
(N=535)<br />
(%)<br />
*<br />
Somnolence also contains: sedation and hypersomnia<br />
†<br />
Decreased appetite also contains: anorexia<br />
‡<br />
Agitation also contains: feeling jittery, nervousness, restlessness, tension, and psychomotor agitation<br />
Adverse events reported at a rate of ≥5% and at least twice the rate of placebo<br />
Data are from two 3-month and two 6-month placebo-controlled studies<br />
Overall discontinuation rate due to adverse events: Cymbalta (20-120 mg/day): 20% vs placebo: 12% 2<br />
Please see Important Safety Information, including Boxed Warning,<br />
for Cymbalta and enclosed full Prescribing Information.<br />
*<br />
*<br />
11<br />
5<br />
4<br />
3<br />
2<br />
1<br />
2<br />
*<br />
Cymbalta 60 mg/day (N=116)<br />
Placebo (N=118)<br />
One outpatient study (MMRM)<br />
* P≤.05, Cymbalta vs placebo<br />
In a separate study, % change in<br />
mean BPI 24-hour average pain<br />
score improvement was measured<br />
at weeks 1, 2, 4, 7, 11, and 151c – Cymbalta 60 mg/day was not<br />
significantly different vs placebo<br />
at weeks 7, 11, and 15<br />
† As measured by an 11-point<br />
Likert Scale<br />
Mean baseline score 6.41b BPI= Brief Pain Inventory<br />
MMRM=Mixed-effects Models<br />
Repeated Measures analysis<br />
% of patients on Cymbalta<br />
who discontinued due<br />
to treatment-emergent<br />
adverse events 1d<br />
1.9<br />
0.1<br />
0.3<br />
2.1<br />
0.1<br />
0.5<br />
0.7<br />
(continued on next page)