the production of thymoquinone from thymol and carvacrol
the production of thymoquinone from thymol and carvacrol
the production of thymoquinone from thymol and carvacrol
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
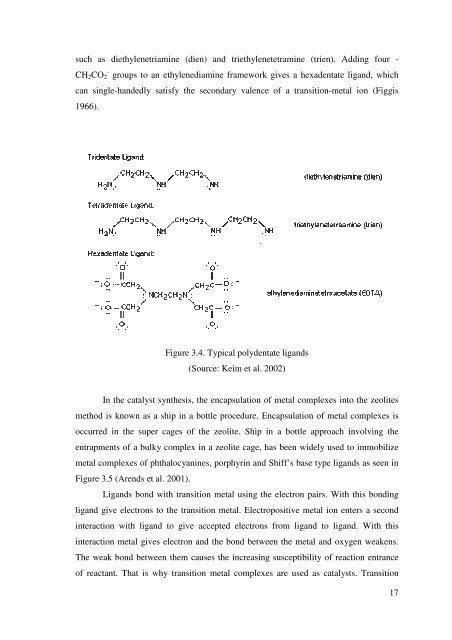
such as diethylenetriamine (dien) <strong>and</strong> triethylenetetramine (trien). Adding four -<br />
CH2CO2 - groups to an ethylenediamine framework gives a hexadentate lig<strong>and</strong>, which<br />
can single-h<strong>and</strong>edly satisfy <strong>the</strong> secondary valence <strong>of</strong> a transition-metal ion (Figgis<br />
1966).<br />
Figure 3.4. Typical polydentate lig<strong>and</strong>s<br />
(Source: Keim et al. 2002)<br />
In <strong>the</strong> catalyst syn<strong>the</strong>sis, <strong>the</strong> encapsulation <strong>of</strong> metal complexes into <strong>the</strong> zeolites<br />
method is known as a ship in a bottle procedure. Encapsulation <strong>of</strong> metal complexes is<br />
occurred in <strong>the</strong> super cages <strong>of</strong> <strong>the</strong> zeolite. Ship in a bottle approach involving <strong>the</strong><br />
entrapments <strong>of</strong> a bulky complex in a zeolite cage, has been widely used to immobilize<br />
metal complexes <strong>of</strong> phthalocyanines, porphyrin <strong>and</strong> Shiff’s base type lig<strong>and</strong>s as seen in<br />
Figure 3.5 (Arends et al. 2001).<br />
Lig<strong>and</strong>s bond with transition metal using <strong>the</strong> electron pairs. With this bonding<br />
lig<strong>and</strong> give electrons to <strong>the</strong> transition metal. Electropositive metal ion enters a second<br />
interaction with lig<strong>and</strong> to give accepted electrons <strong>from</strong> lig<strong>and</strong> to lig<strong>and</strong>. With this<br />
interaction metal gives electron <strong>and</strong> <strong>the</strong> bond between <strong>the</strong> metal <strong>and</strong> oxygen weakens.<br />
The weak bond between <strong>the</strong>m causes <strong>the</strong> increasing susceptibility <strong>of</strong> reaction entrance<br />
<strong>of</strong> reactant. That is why transition metal complexes are used as catalysts. Transition<br />
17