DOH WITH ATTACHED AGENCIES.pdf - University of the ...
DOH WITH ATTACHED AGENCIES.pdf - University of the ...
DOH WITH ATTACHED AGENCIES.pdf - University of the ...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
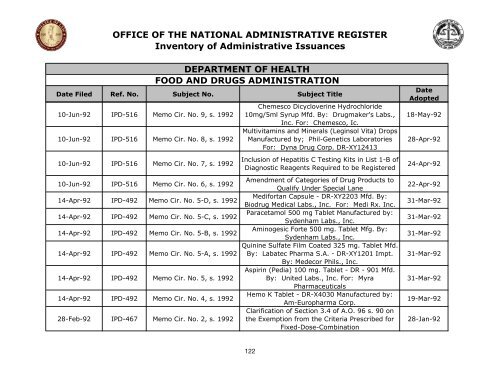
OFFICE OF THE NATIONAL ADMINISTRATIVE REGISTER<br />
Inventory <strong>of</strong> Administrative Issuances<br />
DEPARTMENT OF HEALTH<br />
FOOD AND DRUGS ADMINISTRATION<br />
Date Filed Ref. No. Subject No. Subject Title<br />
10-Jun-92 IPD-516 Memo Cir. No. 9, s. 1992<br />
10-Jun-92 IPD-516 Memo Cir. No. 8, s. 1992<br />
10-Jun-92 IPD-516 Memo Cir. No. 7, s. 1992<br />
10-Jun-92 IPD-516 Memo Cir. No. 6, s. 1992<br />
14-Apr-92 IPD-492 Memo Cir. No. 5-D, s. 1992<br />
14-Apr-92 IPD-492 Memo Cir. No. 5-C, s. 1992<br />
14-Apr-92 IPD-492 Memo Cir. No. 5-B, s. 1992<br />
14-Apr-92 IPD-492 Memo Cir. No. 5-A, s. 1992<br />
14-Apr-92 IPD-492 Memo Cir. No. 5, s. 1992<br />
14-Apr-92 IPD-492 Memo Cir. No. 4, s. 1992<br />
28-Feb-92 IPD-467 Memo Cir. No. 2, s. 1992<br />
Chemesco Dicycloverine Hydrochloride<br />
10mg/5ml Syrup Mfd. By: Drugmaker's Labs.,<br />
Inc. For: Chemesco, Ic.<br />
Multivitamins and Minerals (Leginsol Vita) Drops<br />
Manufactured by; Phil-Genetics Laboratories<br />
For: Dyna Drug Corp. DR-XY12413<br />
Inclusion <strong>of</strong> Hepatitis C Testing Kits in List 1-B <strong>of</strong><br />
Diagnostic Reagents Required to be Registered<br />
Amendment <strong>of</strong> Categories <strong>of</strong> Drug Products to<br />
Qualify Under Special Lane<br />
Medifortan Capsule - DR-XY2203 Mfd. By:<br />
Biodrug Medical Labs., Inc. For: Medi Rx. Inc.<br />
Paracetamol 500 mg Tablet Manufactured by:<br />
Sydenham Labs., Inc.<br />
Aminogesic Forte 500 mg. Tablet Mfg. By:<br />
Sydenham Labs., Inc.<br />
Quinine Sulfate Film Coated 325 mg. Tablet Mfd.<br />
By: Labatec Pharma S.A. - DR-XY1201 Impt.<br />
By: Medecor Phils., Inc.<br />
Aspirin (Pedia) 100 mg. Tablet - DR - 901 Mfd.<br />
By: United Labs., Inc. For: Myra<br />
Pharmaceuticals<br />
Hemo K Tablet - DR-X4030 Manufactured by:<br />
Am-Europharma Corp.<br />
Clarification <strong>of</strong> Section 3.4 <strong>of</strong> A.O. 96 s. 90 on<br />
<strong>the</strong> Exemption from <strong>the</strong> Criteria Prescribed for<br />
Fixed-Dose-Combination<br />
122<br />
Date<br />
Adopted<br />
18-May-92<br />
28-Apr-92<br />
24-Apr-92<br />
22-Apr-92<br />
31-Mar-92<br />
31-Mar-92<br />
31-Mar-92<br />
31-Mar-92<br />
31-Mar-92<br />
19-Mar-92<br />
28-Jan-92