DOH WITH ATTACHED AGENCIES.pdf - University of the ...
DOH WITH ATTACHED AGENCIES.pdf - University of the ...
DOH WITH ATTACHED AGENCIES.pdf - University of the ...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
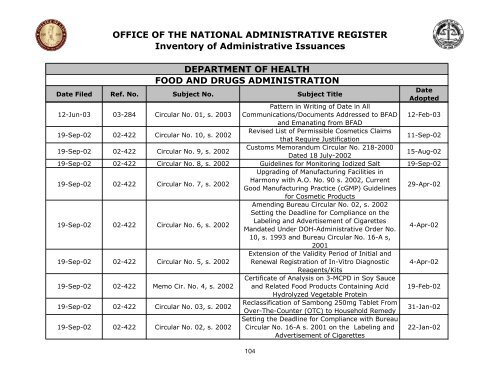
OFFICE OF THE NATIONAL ADMINISTRATIVE REGISTER<br />
Inventory <strong>of</strong> Administrative Issuances<br />
DEPARTMENT OF HEALTH<br />
FOOD AND DRUGS ADMINISTRATION<br />
Date Filed Ref. No. Subject No. Subject Title<br />
Pattern in Writing <strong>of</strong> Date in All<br />
Date<br />
Adopted<br />
12-Jun-03 03-284 Circular No. 01, s. 2003 Communications/Documents Addressed to BFAD<br />
and Emanating from BFAD<br />
12-Feb-03<br />
19-Sep-02 02-422 Circular No. 10, s. 2002<br />
Revised List <strong>of</strong> Permissible Cosmetics Claims<br />
that Require Justification<br />
11-Sep-02<br />
19-Sep-02 02-422 Circular No. 9, s. 2002<br />
Customs Memorandum Circular No. 218-2000<br />
Dated 18 July-2002<br />
15-Aug-02<br />
19-Sep-02 02-422 Circular No. 8, s. 2002 Guidelines for Monitoring Iodized Salt<br />
Upgrading <strong>of</strong> Manufacturing Facilities in<br />
19-Sep-02<br />
19-Sep-02 02-422 Circular No. 7, s. 2002<br />
Harmony with A.O. No. 90 s. 2002, Current<br />
Good Manufacturing Practice (cGMP) Guidelines<br />
for Cosmetic Products<br />
Amending Bureau Circular No. 02, s. 2002<br />
Setting <strong>the</strong> Deadline for Compliance on <strong>the</strong><br />
29-Apr-02<br />
19-Sep-02 02-422 Circular No. 6, s. 2002<br />
Labeling and Advertisement <strong>of</strong> Cigarettes<br />
Mandated Under <strong>DOH</strong>-Administrative Order No.<br />
10, s. 1993 and Bureau Circular No. 16-A s,<br />
2001<br />
Extension <strong>of</strong> <strong>the</strong> Validity Period <strong>of</strong> Initial and<br />
4-Apr-02<br />
19-Sep-02 02-422 Circular No. 5, s. 2002 Renewal Registration <strong>of</strong> In-Vitro Diagnostic<br />
Reagents/Kits<br />
Certificate <strong>of</strong> Analysis on 3-MCPD in Soy Sauce<br />
4-Apr-02<br />
19-Sep-02 02-422 Memo Cir. No. 4, s. 2002 and Related Food Products Containing Acid<br />
Hydrolyzed Vegetable Protein<br />
19-Feb-02<br />
19-Sep-02 02-422 Circular No. 03, s. 2002<br />
Reclassification <strong>of</strong> Sambong 250mg Tablet From<br />
Over-The-Counter (OTC) to Household Remedy<br />
Setting <strong>the</strong> Deadline for Compliance with Bureau<br />
31-Jan-02<br />
19-Sep-02 02-422 Circular No. 02, s. 2002 Circular No. 16-A s. 2001 on <strong>the</strong> Labeling and<br />
Advertisement <strong>of</strong> Cigarettes<br />
22-Jan-02<br />
104