DOH WITH ATTACHED AGENCIES.pdf - University of the ...
DOH WITH ATTACHED AGENCIES.pdf - University of the ...
DOH WITH ATTACHED AGENCIES.pdf - University of the ...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
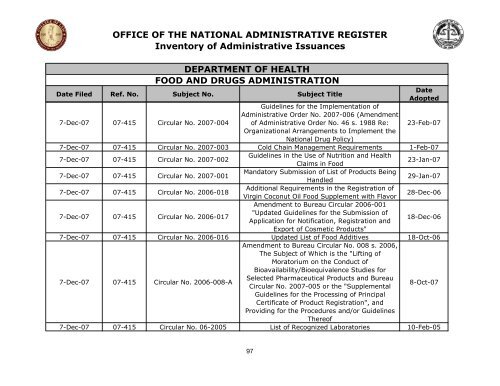
OFFICE OF THE NATIONAL ADMINISTRATIVE REGISTER<br />
Inventory <strong>of</strong> Administrative Issuances<br />
DEPARTMENT OF HEALTH<br />
FOOD AND DRUGS ADMINISTRATION<br />
Date Filed Ref. No. Subject No. Subject Title<br />
Guidelines for <strong>the</strong> Implementation <strong>of</strong><br />
Administrative Order No. 2007-006 (Amendment<br />
Date<br />
Adopted<br />
7-Dec-07 07-415 Circular No. 2007-004 <strong>of</strong> Administrative Order No. 46 s. 1988 Re:<br />
Organizational Arrangements to Implement <strong>the</strong><br />
National Drug Policy)<br />
23-Feb-07<br />
7-Dec-07 07-415 Circular No. 2007-003 Cold Chain Management Requirements 1-Feb-07<br />
7-Dec-07 07-415 Circular No. 2007-002<br />
Guidelines in <strong>the</strong> Use <strong>of</strong> Nutrition and Health<br />
Claims in Food<br />
23-Jan-07<br />
7-Dec-07 07-415 Circular No. 2007-001<br />
Mandatory Submission <strong>of</strong> List <strong>of</strong> Products Being<br />
Handled<br />
29-Jan-07<br />
7-Dec-07 07-415 Circular No. 2006-018<br />
Additional Requirements in <strong>the</strong> Registration <strong>of</strong><br />
Virgin Coconut Oil Food Supplement with Flavor<br />
Amendment to Bureau Circular 2006-001<br />
28-Dec-06<br />
7-Dec-07 07-415 Circular No. 2006-017<br />
"Updated Guidelines for <strong>the</strong> Submission <strong>of</strong><br />
Application for Notification, Registration and<br />
Export <strong>of</strong> Cosmetic Products"<br />
18-Dec-06<br />
7-Dec-07 07-415 Circular No. 2006-016 Updated List <strong>of</strong> Food Additives<br />
Amendment to Bureau Circular No. 008 s. 2006,<br />
The Subject <strong>of</strong> Which is <strong>the</strong> "Lifting <strong>of</strong><br />
Moratorium on <strong>the</strong> Conduct <strong>of</strong><br />
Bioavailability/Bioequivalence Studies for<br />
18-Oct-06<br />
7-Dec-07 07-415 Circular No. 2006-008-A<br />
Selected Pharmaceutical Products and Bureau<br />
Circular No. 2007-005 or <strong>the</strong> "Supplemental<br />
Guidelines for <strong>the</strong> Processing <strong>of</strong> Principal<br />
Certificate <strong>of</strong> Product Registration", and<br />
Providing for <strong>the</strong> Procedures and/or Guidelines<br />
There<strong>of</strong><br />
8-Oct-07<br />
7-Dec-07 07-415 Circular No. 06-2005 List <strong>of</strong> Recognized Laboratories 10-Feb-05<br />
97