Prions: Protein Aggregation and Infectious Diseases - Physiological ...
Prions: Protein Aggregation and Infectious Diseases - Physiological ...
Prions: Protein Aggregation and Infectious Diseases - Physiological ...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
merized into rod-shaped particles with an ultrastructural<br />
appearance of amyloid (Fig. 1C) containing 50% -sheet<br />
<strong>and</strong> 20% -helix content (103, 176, 435). In contrast to<br />
PrP 27–30 , neither purified PrP C nor PrP Sc forms aggregates<br />
detectable by electron microscopy (338, 379).<br />
2. Models describing the replication of prions<br />
Transgenic mouse studies have provided genetic<br />
(412) <strong>and</strong> biochemical (343) evidence that the conversion<br />
of PrP C to PrP Sc occurs through the formation of a PrP C /<br />
PrP Sc complex. However, such a complex has never been<br />
isolated to purity. Consequently, it is unclear whether<br />
PrP C binds to one or more additional macromolecules<br />
during the process of PrP C to PrP Sc conversion. The formation<br />
of a PrP C /PrP Sc complex may be facilitated when<br />
the amino acid sequences of the two PrP isoforms are<br />
identical. For instance, hamster PrP differs from mouse<br />
PrP at 16 of 254 positions, perhaps explaining the strong<br />
species barrier between mice <strong>and</strong> hamsters. Differences<br />
in amino acid sequence delay the onset of prion disease<br />
by prolonging the incubation time (446). As the sequence<br />
specificity of PrP Sc formation is more important for the<br />
conversion to protease resistance than for the initial binding<br />
steps, additional intermolecular interactions between<br />
PrP C <strong>and</strong> PrP Sc may be required to complete the process<br />
of conversion to the protease-resistant state (228).<br />
The level of PrP C expression appears to be directly<br />
proportional to the rate of PrP Sc formation <strong>and</strong>, thus, inversely<br />
related to the length of the incubation time. Prnp o/o<br />
mice are resistant to prions <strong>and</strong> do not propagate scrapie<br />
infectivity (84, 407, 439). Mice hemizygous (Prnp /o ) for<br />
ablation of the Prnp gene have prolonged incubation times<br />
when inoculated with mouse prions (83, 326).<br />
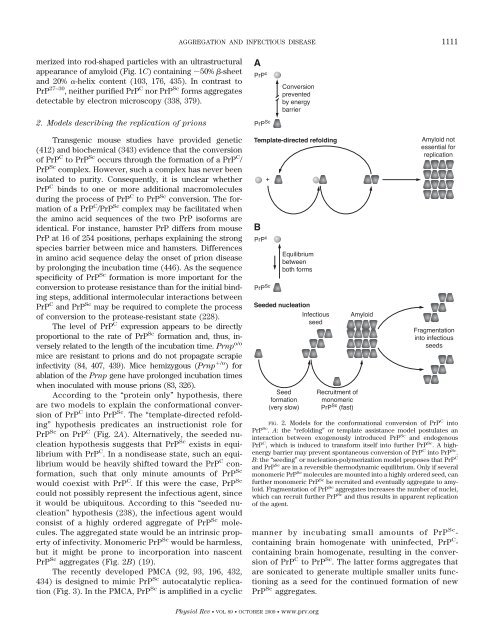
According to the “protein only” hypothesis, there<br />
are two models to explain the conformational conversion<br />
of PrP C into PrP Sc . The “template-directed refolding”<br />
hypothesis predicates an instructionist role for<br />
PrP Sc on PrP C (Fig. 2A). Alternatively, the seeded nucleation<br />
hypothesis suggests that PrP Sc exists in equilibrium<br />
with PrP C . In a nondisease state, such an equilibrium<br />
would be heavily shifted toward the PrP C conformation,<br />
such that only minute amounts of PrP Sc<br />
would coexist with PrP C . If this were the case, PrP Sc<br />
could not possibly represent the infectious agent, since<br />
it would be ubiquitous. According to this “seeded nucleation”<br />
hypothesis (238), the infectious agent would<br />
consist of a highly ordered aggregate of PrP Sc molecules.<br />
The aggregated state would be an intrinsic property<br />
of infectivity. Monomeric PrP Sc would be harmless,<br />
but it might be prone to incorporation into nascent<br />
PrP Sc aggregates (Fig. 2B) (19).<br />
The recently developed PMCA (92, 93, 196, 432,<br />
434) is designed to mimic PrP Sc autocatalytic replication<br />
(Fig. 3). In the PMCA, PrP Sc is amplified in a cyclic<br />
AGGREGATION AND INFECTIOUS DISEASE 1111<br />
Physiol Rev VOL 89 OCTOBER 2009 www.prv.org<br />
FIG. 2. Models for the conformational conversion of PrP C into<br />
PrP Sc . A: the “refolding” or template assistance model postulates an<br />
interaction between exogenously introduced PrP Sc <strong>and</strong> endogenous<br />
PrP C , which is induced to transform itself into further PrP Sc . A highenergy<br />
barrier may prevent spontaneous conversion of PrP C into PrP Sc .<br />
B: the “seeding” or nucleation-polymerization model proposes that PrP C<br />
<strong>and</strong> PrP Sc are in a reversible thermodynamic equilibrium. Only if several<br />
monomeric PrP Sc molecules are mounted into a highly ordered seed, can<br />
further monomeric PrP Sc be recruited <strong>and</strong> eventually aggregate to amyloid.<br />
Fragmentation of PrP Sc aggregates increases the number of nuclei,<br />
which can recruit further PrP Sc <strong>and</strong> thus results in apparent replication<br />
of the agent.<br />
manner by incubating small amounts of PrP Sc -<br />
containing brain homogenate with uninfected, PrP C -<br />
containing brain homogenate, resulting in the conversion<br />
of PrP C to PrP Sc . The latter forms aggregates that<br />
are sonicated to generate multiple smaller units functioning<br />
as a seed for the continued formation of new<br />
PrP Sc aggregates.