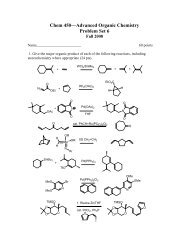
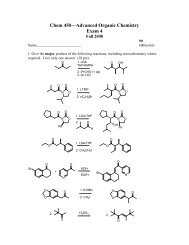
Chemistry 355
Chemistry 355
Chemistry 355
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
<strong>Chemistry</strong> 100<br />
Reactions and Reason<br />
Spring 2011<br />
Quiz 3<br />
Name:_______________________ January 28, 2011<br />
A periodic table and conversion factors are on the back of this quiz. You must show your<br />
work for full credit.<br />
1. Give the following for the element carbon.<br />
a. Symbol ______________C_________________________<br />
b. Atomic Number ______________6__________________________<br />
c. Number of electrons ______________6__________________________<br />
2. Give the following for the element K<br />
a. Name ________Potassium_______________________<br />
b. Atomic weight _________39.098 amu_____________________<br />
c. Number of protons ___________19___________________________<br />
3. How many grams are in a five pound bag of sugar?<br />
5 lb | 454 g = 2270 g<br />
| 1 lb
1<br />
IA<br />
1<br />
H<br />
1.0079<br />
3<br />
Li<br />
6.941<br />
19<br />
K<br />
39.098<br />
37<br />
Rb<br />
85.468<br />
55<br />
Cs<br />
132.91<br />
87<br />
Fr<br />
(223)<br />
2<br />
IIA<br />
4<br />
Be<br />
9.0122<br />
20<br />
Ca<br />
40.078<br />
38<br />
Sr<br />
87.62<br />
56<br />
Ba<br />
137.33<br />
57<br />
* La<br />
88 89 104<br />
Ra ** Ac Rf<br />
(226)<br />
21<br />
Sc<br />
44.956<br />
39<br />
Y<br />
88.906<br />
138.91<br />
227.03<br />
22<br />
Ti<br />
47.88<br />
40<br />
Zr<br />
91.224<br />
72<br />
Hf<br />
178.49<br />
(261)<br />
*Lanthanides<br />
**Actinides<br />
Periodic Table of the Elements<br />
23<br />
V<br />
50.942<br />
41<br />
Nb<br />
92.906<br />
73<br />
Ta<br />
180.95<br />
105<br />
Ha<br />
(262)<br />
58<br />
Ce<br />
140.12<br />
90<br />
Th<br />
232.04<br />
24<br />
Cr<br />
51.996<br />
42<br />
Mo<br />
95.94<br />
74<br />
W<br />
183.85<br />
106<br />
Sg<br />
(263)<br />
59<br />
Pr<br />
140.91<br />
91<br />
Pa<br />
231.04<br />
25<br />
Mn<br />
54.938<br />
43<br />
Tc<br />
(98)<br />
75<br />
Re<br />
186.21<br />
107<br />
Ns<br />
(262)<br />
60<br />
Nd<br />
144.24<br />
92<br />
U<br />
238.03<br />
26<br />
Fe<br />
55.845<br />
44<br />
Ru<br />
101.07<br />
76<br />
Os<br />
190.2<br />
108<br />
Hs<br />
(265)<br />
61<br />
Pm<br />
(145)<br />
93<br />
Np<br />
237.05<br />
27<br />
Co<br />
45<br />
Rh<br />
102.91<br />
77<br />
Ir<br />
192.22<br />
109<br />
Mt<br />
(266)<br />
62<br />
Sm<br />
150.36<br />
63<br />
Eu<br />
64<br />
Gd<br />
65<br />
Tb<br />
13 14<br />
IIIA IVA<br />
66<br />
Dy<br />
67<br />
Ho<br />
94 95 96 97 98 99<br />
Pu Am Cm Bk Cf Es<br />
(244)<br />
28<br />
Ni<br />
46<br />
Pd<br />
106.42<br />
78<br />
Pt<br />
195.08<br />
(269)<br />
151.97<br />
(243)<br />
29<br />
Cu<br />
47<br />
Ag<br />
107.87<br />
79<br />
Au<br />
196.97<br />
(272)<br />
157.25<br />
(247)<br />
30<br />
Zn<br />
48<br />
Cd<br />
112.41<br />
80<br />
Hg<br />
200.59<br />
110 111 112<br />
Uun Uuu Uub<br />
(277)<br />
158.93<br />
(247)<br />
5<br />
B<br />
10.811<br />
31<br />
Ga<br />
58.933 58.693 63.546 65.39 69.723<br />
49<br />
In<br />
114.82<br />
81<br />
Tl<br />
204.38<br />
162.50<br />
(251)<br />
6<br />
C<br />
12.011<br />
11 12 13 14<br />
Na<br />
22.990<br />
Mg<br />
24.305<br />
3 4 5 6 7 8 9 10 11 12<br />
IIIB IVB VB VIB VIIB VIIB IB IIB<br />
Al<br />
26.982<br />
Si<br />
28.086<br />
Conversion Factors/Constants<br />
1 pound = 16 ounces 1 pound = 454 grams 1 kilometer = 0.6214 miles<br />
2.2 pounds = 1 kilogram 1 foot = 12 inches 1 inch = 2.54 centimeters<br />
1 yard = 3 feet 1 yard = 0.91 meters 1 mile = 5280 feet<br />
1 gallon = 4 quarts 1 gallon = 3.78 liters 1 day = 24 hours<br />
1 hour = 60 minutes 1 minute = 60 s 1 year = 365 days<br />
1 Joule = 1 kg m 2 /s 2 c = 2.9979 x 10 8 m/s h = 6.626 x 10 -34 J s<br />
NA=6.022 x 10 23 SHWater=1.00 cal/g o C Kw = 1.00 x 10 -14<br />
32<br />
Ge<br />
72.61<br />
50<br />
Sn<br />
118.71<br />
82<br />
Pb<br />
207.2<br />
164.93<br />
(252)