CHEMISTRY 314-01 MIDTERM # 1 – answer key September 29 ...
CHEMISTRY 314-01 MIDTERM # 1 – answer key September 29 ...
CHEMISTRY 314-01 MIDTERM # 1 – answer key September 29 ...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
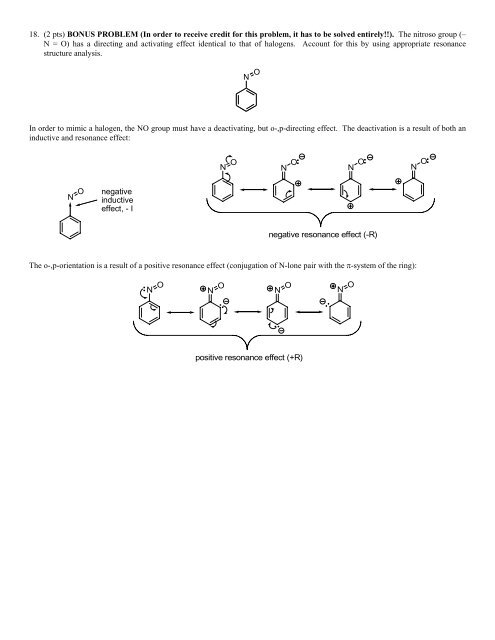
18. (2 pts) BONUS PROBLEM (In order to receive credit for this problem, it has to be solved entirely!!). The nitroso group (<strong>–</strong><br />
N = O) has a directing and activating effect identical to that of halogens. Account for this by using appropriate resonance<br />
structure analysis.<br />
N O<br />
In order to mimic a halogen, the NO group must have a deactivating, but o-,p-directing effect. The deactivation is a result of both an<br />
inductive and resonance effect:<br />
N<br />
O negative<br />
inductive<br />
effect, - I<br />
N O<br />
N O<br />
N O<br />
negative resonance effect (-R)<br />
The o-,p-orientation is a result of a positive resonance effect (conjugation of N-lone pair with the π-system of the ring):<br />
N O<br />
N O<br />
N O<br />
positive resonance effect (+R)<br />
N O<br />
N O