Voie d'immunisation et séquence d'administration de l ... - TEL
Voie d'immunisation et séquence d'administration de l ... - TEL Voie d'immunisation et séquence d'administration de l ... - TEL
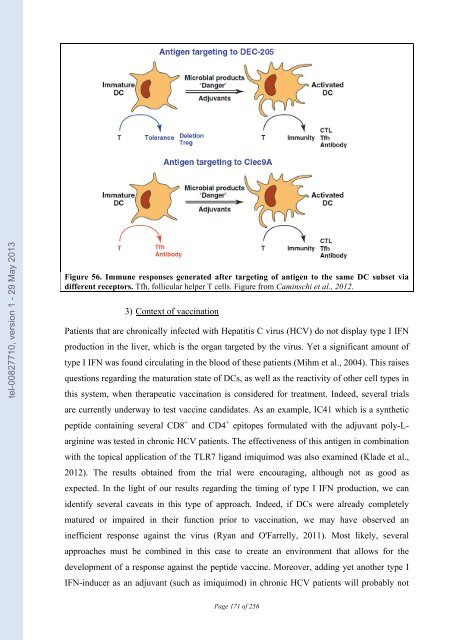
tel-00827710, version 1 - 29 May 2013 of monocytes into DCs with varying phenotypes and functions. Activation of monocytes with IL-4, IFNα, TNF or IL-15 gave rise to different populations of DCs with diverse phenotypes. That could explain the different efficiencies of these cells in mounting CD8 + T cell responses (Paquette et al., 1998; Dubsky et al., 2007). (b) Side effects of type I IFN treatment on DCs Not all type I IFN effects on DCs promote a well-regulated immune response. Autoimmune disorders have been observed in melanoma patients undergoing treatment with type I IFN. One hypothesis is that IFN induced in vivo maturation of DCs that have taken up self-antigen and this led to an autoimmune response (Rizza et al., 2010). (c) Targeting DCs with specific antigens It was previously introduced that the type of antigen and thus the type of receptor implicated for antigen uptake plays a role in the efficiency of the subsequent immune response. One promising approach for vaccination development is to target antigens to a specific subset of DCs by coupling them with antibodies directed against DC-specific cell-surface molecules. The CD8α + DC subset has been the most studied thus far, due to its specialization for cross- priming. Antibodies coupled to Ovalbumin and targeting different receptors, DEC-205, Clec9A and Clec12A were tested. Interestingly it was observed that the outcome of the response and the requirement for adjuvant depends on which receptor was targeted. DEC-205 and Clec9A are effective targets to promote cytotoxic T cell responses, while Clec12A was shown to be inefficient (Lahoud et al., 2011). Addition of adjuvant is always required to induce a CD8 + T cell response. In contrast, a potent humoral response can be obtained upon targeting of antigen to Clec9A in the absence of adjuvant (Figure 56). Since Clec9A expression is restricted to CD8α + DCs and pDCs, this observation may be due to a longer persistence of antigen coupled to antibody in the blood because fewer cells are available that can endocytosed it, allowing for a sustained presentation on MHC-II. 170
tel-00827710, version 1 - 29 May 2013 Figure 56. Immune responses generated after targeting of antigen to the same DC subset via different receptors. Tfh, follicular helper T cells. Figure from Caminschi et al., 2012. 3) Context of vaccination Patients that are chronically infected with Hepatitis C virus (HCV) do not display type I IFN production in the liver, which is the organ targeted by the virus. Yet a significant amount of type I IFN was found circulating in the blood of these patients (Mihm et al., 2004). This raises questions regarding the maturation state of DCs, as well as the reactivity of other cell types in this system, when therapeutic vaccination is considered for treatment. Indeed, several trials are currently underway to test vaccine candidates. As an example, IC41 which is a synthetic peptide containing several CD8 + and CD4 + epitopes formulated with the adjuvant poly-L- arginine was tested in chronic HCV patients. The effectiveness of this antigen in combination with the topical application of the TLR7 ligand imiquimod was also examined (Klade et al., 2012). The results obtained from the trial were encouraging, although not as good as expected. In the light of our results regarding the timing of type I IFN production, we can identify several caveats in this type of approach. Indeed, if DCs were already completely matured or impaired in their function prior to vaccination, we may have observed an inefficient response against the virus (Ryan and O'Farrelly, 2011). Most likely, several approaches must be combined in this case to create an environment that allows for the development of a response against the peptide vaccine. Moreover, adding yet another type I IFN-inducer as an adjuvant (such as imiquimod) in chronic HCV patients will probably not Page 171 of 256
- Page 119 and 120: tel-00827710, version 1 - 29 May 20
- Page 121 and 122: tel-00827710, version 1 - 29 May 20
- Page 123 and 124: tel-00827710, version 1 - 29 May 20
- Page 125 and 126: tel-00827710, version 1 - 29 May 20
- Page 127 and 128: tel-00827710, version 1 - 29 May 20
- Page 129 and 130: tel-00827710, version 1 - 29 May 20
- Page 131 and 132: tel-00827710, version 1 - 29 May 20
- Page 133 and 134: tel-00827710, version 1 - 29 May 20
- Page 135 and 136: tel-00827710, version 1 - 29 May 20
- Page 137 and 138: tel-00827710, version 1 - 29 May 20
- Page 139 and 140: tel-00827710, version 1 - 29 May 20
- Page 141 and 142: tel-00827710, version 1 - 29 May 20
- Page 143 and 144: tel-00827710, version 1 - 29 May 20
- Page 145 and 146: tel-00827710, version 1 - 29 May 20
- Page 147 and 148: tel-00827710, version 1 - 29 May 20
- Page 149 and 150: tel-00827710, version 1 - 29 May 20
- Page 151 and 152: tel-00827710, version 1 - 29 May 20
- Page 153 and 154: tel-00827710, version 1 - 29 May 20
- Page 155 and 156: tel-00827710, version 1 - 29 May 20
- Page 157 and 158: tel-00827710, version 1 - 29 May 20
- Page 159 and 160: tel-00827710, version 1 - 29 May 20
- Page 161 and 162: tel-00827710, version 1 - 29 May 20
- Page 163 and 164: tel-00827710, version 1 - 29 May 20
- Page 165 and 166: tel-00827710, version 1 - 29 May 20
- Page 167 and 168: tel-00827710, version 1 - 29 May 20
- Page 169: tel-00827710, version 1 - 29 May 20
- Page 173 and 174: tel-00827710, version 1 - 29 May 20
- Page 175 and 176: tel-00827710, version 1 - 29 May 20
- Page 177 and 178: tel-00827710, version 1 - 29 May 20
- Page 179 and 180: tel-00827710, version 1 - 29 May 20
- Page 181 and 182: tel-00827710, version 1 - 29 May 20
- Page 183 and 184: tel-00827710, version 1 - 29 May 20
- Page 185 and 186: tel-00827710, version 1 - 29 May 20
- Page 187 and 188: tel-00827710, version 1 - 29 May 20
- Page 189 and 190: tel-00827710, version 1 - 29 May 20
- Page 191 and 192: tel-00827710, version 1 - 29 May 20
- Page 193 and 194: tel-00827710, version 1 - 29 May 20
- Page 195 and 196: tel-00827710, version 1 - 29 May 20
- Page 197 and 198: tel-00827710, version 1 - 29 May 20
- Page 199 and 200: tel-00827710, version 1 - 29 May 20
- Page 201 and 202: tel-00827710, version 1 - 29 May 20
- Page 203 and 204: tel-00827710, version 1 - 29 May 20
- Page 205 and 206: tel-00827710, version 1 - 29 May 20
- Page 207 and 208: tel-00827710, version 1 - 29 May 20
- Page 209 and 210: tel-00827710, version 1 - 29 May 20
- Page 211 and 212: tel-00827710, version 1 - 29 May 20
- Page 213 and 214: tel-00827710, version 1 - 29 May 20
- Page 215 and 216: tel-00827710, version 1 - 29 May 20
- Page 217 and 218: tel-00827710, version 1 - 29 May 20
- Page 219 and 220: tel-00827710, version 1 - 29 May 20
tel-00827710, version 1 - 29 May 2013<br />
Figure 56. Immune responses generated after targ<strong>et</strong>ing of antigen to the same DC subs<strong>et</strong> via<br />
different receptors. Tfh, follicular helper T cells. Figure from Caminschi <strong>et</strong> al., 2012.<br />
3) Context of vaccination<br />
Patients that are chronically infected with Hepatitis C virus (HCV) do not display type I IFN<br />
production in the liver, which is the organ targ<strong>et</strong>ed by the virus. Y<strong>et</strong> a significant amount of<br />
type I IFN was found circulating in the blood of these patients (Mihm <strong>et</strong> al., 2004). This raises<br />
questions regarding the maturation state of DCs, as well as the reactivity of other cell types in<br />
this system, when therapeutic vaccination is consi<strong>de</strong>red for treatment. In<strong>de</strong>ed, several trials<br />
are currently un<strong>de</strong>rway to test vaccine candidates. As an example, IC41 which is a synth<strong>et</strong>ic<br />
pepti<strong>de</strong> containing several CD8 + and CD4 + epitopes formulated with the adjuvant poly-L-<br />
arginine was tested in chronic HCV patients. The effectiveness of this antigen in combination<br />
with the topical application of the TLR7 ligand imiquimod was also examined (Kla<strong>de</strong> <strong>et</strong> al.,<br />
2012). The results obtained from the trial were encouraging, although not as good as<br />
expected. In the light of our results regarding the timing of type I IFN production, we can<br />
i<strong>de</strong>ntify several caveats in this type of approach. In<strong>de</strong>ed, if DCs were already compl<strong>et</strong>ely<br />
matured or impaired in their function prior to vaccination, we may have observed an<br />
inefficient response against the virus (Ryan and O'Farrelly, 2011). Most likely, several<br />
approaches must be combined in this case to create an environment that allows for the<br />
<strong>de</strong>velopment of a response against the pepti<strong>de</strong> vaccine. Moreover, adding y<strong>et</strong> another type I<br />
IFN-inducer as an adjuvant (such as imiquimod) in chronic HCV patients will probably not<br />
Page 171 of 256