Protolytic equilibrium in lyophilic nanosized dispersions ... - IUPAC
Protolytic equilibrium in lyophilic nanosized dispersions ... - IUPAC
Protolytic equilibrium in lyophilic nanosized dispersions ... - IUPAC
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
<strong>Protolytic</strong> <strong>equilibrium</strong> <strong>in</strong> <strong>lyophilic</strong> <strong>dispersions</strong> 1471<br />
Ψ = 59.16 ( pKi a – pKac a ); 25 °C (16)<br />
The pKi a value <strong>in</strong> ionic micelles is often equated to the pK ac<br />
a value of the same <strong>in</strong>dicator bound by<br />
micelles of nonionic surfactants [17e,h,28l,29c,d,i,60e]. For example, with Reichardt’s <strong>in</strong>dicator<br />
(Table 1), one can estimate the Ψ values of –95 and +99 mV for SDS and CTAB micelles at I = 0.05 M,<br />
us<strong>in</strong>g the pKi a value of 9.10.<br />
Based on an equation similar to eq. 16, Tokiwa and Ohki [65b] developed a method, which allowed<br />
estimat<strong>in</strong>g of the Ψ values of dimethyl dodecylam<strong>in</strong>e oxide micelles dur<strong>in</strong>g potentiometric titration<br />
of this amphiphilic base with HCl. Funasaki successfully utilized these data <strong>in</strong> his studies with <strong>in</strong>dicators<br />
[60a]. Note that the degree of b<strong>in</strong>d<strong>in</strong>g of counterions, Cl – , can vary along the titration curve.<br />
Therefore, the relation between the degree of protonation of dimethyl dodecylam<strong>in</strong>e oxide and the Ψ<br />
value can be complicated.<br />
In the classical version of the electrostatic model, the constancy of (i) the pKi a value of the given<br />
<strong>in</strong>dicator <strong>in</strong> any micellar system on the one hand, and of (ii) the value of electrostatic potential of the<br />
Stern layer of the given micellar surface as obta<strong>in</strong>ed by us<strong>in</strong>g any <strong>in</strong>dicator, on the other hand, is assumed.<br />
Consequently, the change <strong>in</strong> pK ac<br />
a on go<strong>in</strong>g from one k<strong>in</strong>d of micelles to another must be equal<br />
for all the <strong>in</strong>dicators.<br />
The common electrostatic model of acid–base <strong>in</strong>dicators equilibria <strong>in</strong> micelles, based on the<br />
HMFF equation, is certa<strong>in</strong>ly adequate <strong>in</strong> outl<strong>in</strong>e. However, the two aforementioned assumptions are<br />
proved to be justified only approximately, and sometimes are even <strong>in</strong>valid. In the cited papers, sulfonephthale<strong>in</strong>s,<br />
phenols, azo dyes, coumar<strong>in</strong>es, hydroxyxanthenes, antraqu<strong>in</strong>ones, imidazoles, az<strong>in</strong>es, triarylcarb<strong>in</strong>ols,<br />
and some other dyes were studied. The more number of <strong>in</strong>dicator dyes are <strong>in</strong>volved <strong>in</strong> the<br />
determ<strong>in</strong>ation of Ψ, the stronger discrepancies becomes evident.<br />
Of course, the limited validity of the simple model can reflect the peculiar of dye location with<strong>in</strong><br />
the pseudophase. Indeed, some relatively large-sized dye molecules (ions) cannot completely come <strong>in</strong>to<br />
the Stern region. On the other hand, some extremely hydrophobic dyes can penetrate even <strong>in</strong>to the micellar<br />
or microdroplet core [25,46m]. In the context of the approach under discussion, all these peculiarities<br />
are <strong>in</strong>cluded <strong>in</strong> the wγ m<br />
i values. The electrical potential with<strong>in</strong> the charged uniform sphere stays<br />
constant; however, the existence of local charges and complicated potential profiles <strong>in</strong> the <strong>in</strong>terfacial regions<br />
(micelles of zwitterionic surfactants, phospholipid vesicles, etc.) also results <strong>in</strong> different medium<br />
effects (∆pKac a ) for various <strong>in</strong>dicator dyes.<br />
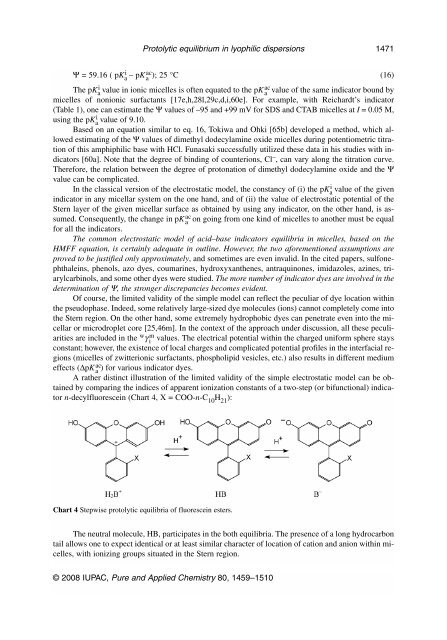
A rather dist<strong>in</strong>ct illustration of the limited validity of the simple electrostatic model can be obta<strong>in</strong>ed<br />
by compar<strong>in</strong>g the <strong>in</strong>dices of apparent ionization constants of a two-step (or bifunctional) <strong>in</strong>dicator<br />
n-decylfluoresce<strong>in</strong> (Chart 4, X = COO-n-C10H21 ):<br />
Chart 4 Stepwise protolytic equilibria of fluoresce<strong>in</strong> esters.<br />
The neutral molecule, HB, participates <strong>in</strong> the both equilibria. The presence of a long hydrocarbon<br />
tail allows one to expect identical or at least similar character of location of cation and anion with<strong>in</strong> micelles,<br />
with ioniz<strong>in</strong>g groups situated <strong>in</strong> the Stern region.<br />
© 2008 <strong>IUPAC</strong>, Pure and Applied Chemistry 80, 1459–1510