Alaska Tuberculosis Program Manual - Epidemiology - State of Alaska
Alaska Tuberculosis Program Manual - Epidemiology - State of Alaska Alaska Tuberculosis Program Manual - Epidemiology - State of Alaska
Antituberculosis Drug Ethambutol (EMB) Rifamate® (INH and RIF) Rifater® (INH, RIF, PZA) Side Effects/ Adverse Reactions Optic neuritis Rash See comments under individual drugs above Monitoring Comments Baseline tests of visual acuity (Snellen chart) and color discrimination (Ishihara tests) At each monthly visit, patients should be questioned regarding possible visual disturbances, including blurred vision or scotomata Monthly testing of visual acuity and color discrimination is recommended for Patients taking doses >15–25 mg/kg Patients receiving EMB for >2 months Patients with renal insufficiency ALASKA T U B E R C U L O S I S P R O G R A M M A N U A L Treatment of Tuber culosis Disease 6.19 Revised November 2012 Optic neuritis may be unilateral; check each eye separately. Patients should be instructed to contact their physician or public health clinic immediately if they experience a change in vision. EMB should be discontinued immediately and permanently if there are any signs of visual toxicity. Definitions of abbreviations: ALT = alanine aminotransferase; AST = aspartate aminotransferase; EMB = ethambutol; HIV = human immunodeficiency virus; INH = isoniazid; NNRTIs = nonnucleoside reverse transcriptase inhibitors; PZA = pyrazinamide; PIs = protease inhibitors; RFB = rifabutin; RIF = rifampin; RPT = rifapentine. Sources: CDC. Targeted tuberculin testing and treatment of latent tuberculosis infection. MMWR 2000;49 (No. RR-6):26–29, 38–39; ATS, CDC, IDSA. Treatment of tuberculosis. MMWR 2003;52(No. RR-11):19–25; CDC. Update: Adverse event data and revised American Thoracic Society/CDC recommendations against the use of rifampin and pyrazinamide for treatment of latent tuberculosis infection—United States. MMWR 2003;52(No. 31):735–736; CDC. : Chapter 6: treatment of TB disease. Core Curriculum on Tuberculosis (2004) [Division of Tuberculosis Elimination Web site]. Updated October 2004. Available at: http://www.cdc.gov/tb/webcourses/CoreCurr/index.htm, Accessed December 30, 2010.
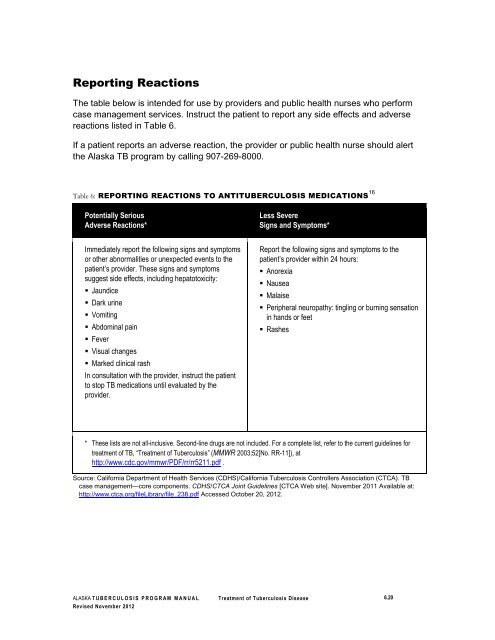
Reporting Reactions The table below is intended for use by providers and public health nurses who perform case management services. Instruct the patient to report any side effects and adverse reactions listed in Table 6. If a patient reports an adverse reaction, the provider or public health nurse should alert the Alaska TB program by calling 907-269-8000. Table 6: REPORTING REACTIONS TO ANTITUBERCULOSIS MEDICATIONS 16 Potentially Serious Adverse Reactions* Immediately report the following signs and symptoms or other abnormalities or unexpected events to the patient’s provider. These signs and symptoms suggest side effects, including hepatotoxicity: Jaundice Dark urine Vomiting Abdominal pain Fever Visual changes Marked clinical rash In consultation with the provider, instruct the patient to stop TB medications until evaluated by the provider. Less Severe Signs and Symptoms* Report the following signs and symptoms to the patient’s provider within 24 hours: Anorexia Nausea Malaise Peripheral neuropathy: tingling or burning sensation in hands or feet Rashes * These lists are not all-inclusive. Second-line drugs are not included. For a complete list, refer to the current guidelines for treatment of TB, “Treatment of Tuberculosis” (MMWR 2003;52[No. RR-11]), at http://www.cdc.gov/mmwr/PDF/rr/rr5211.pdf . Source: California Department of Health Services (CDHS)/California Tuberculosis Controllers Association (CTCA). TB case management—core components. CDHS/CTCA Joint Guidelines [CTCA Web site]. November 2011 Available at: http://www.ctca.org/fileLibrary/file_238.pdf Accessed October 20, 2012. ALASKA T U B E R C U L O S I S P R O G R A M M A N U A L Treatment of Tuber culosis Disease 6.20 Revised November 2012
- Page 59 and 60: Table 1: NUMBERS OF FOREIGN-BORN PE
- Page 61 and 62: chest radiograph and if sputum AFB
- Page 63 and 64: Follow-up of B1 and B2 Tuberculosis
- Page 65 and 66: Evaluation of B1, B2, and B Tubercu
- Page 67 and 68: Treatment Prescribe medications as
- Page 69 and 70: 12 Centers for Disease Control and
- Page 71 and 72: Introduction Purpose Use this secti
- Page 73 and 74: Tuberculosis Classification System
- Page 75 and 76: Table 2: PERSONS AT HIGH RISK FOR T
- Page 77 and 78: Table 3: WHEN TO SUSPECT PULMONARY
- Page 79 and 80: Diagnosis of Tuberculosis Disease T
- Page 81 and 82: 1. Exposure to Infectious TB: Ask p
- Page 83 and 84: Physical Examination A physical exa
- Page 85 and 86: For more information on chest radio
- Page 87 and 88: Laboratories should report positive
- Page 89 and 90: Guidelines for preventing the trans
- Page 91 and 92: 50 CDC. National plan for reliable
- Page 93 and 94: Introduction Purpose The overall go
- Page 95 and 96: Basic Treatment Principles Follow t
- Page 97 and 98: Treatment Regimens and Dosages Use
- Page 99 and 100: Table 3: FOUR TREATMENT REGIMENS FO
- Page 101 and 102: † 4 Table 4: DOSES*OF FIRST-LINE
- Page 103 and 104: Figure 1:TREATMENT ALGORITHM FOR DR
- Page 105 and 106: a. In remote locations in Alaska, m
- Page 107 and 108: Antituberculosis Drug Rifampin (RIF
- Page 109: Antituberculosis Drug Rifapentine (
- Page 113 and 114: Response to Treatment For consultat
- Page 115 and 116: Figure 2: MANAGEMENT OF TREATMENT I
- Page 117 and 118: Post-Treatment Evaluation Routine f
- Page 119 and 120: Treatment in Special Situations Tre
- Page 121 and 122: Resources For consultation regardin
- Page 123 and 124: with an increase in overall complet
- Page 125 and 126: Liver Disease Management of TB in p
- Page 127 and 128: After the initial phase (first two
- Page 129 and 130: Extrapulmonary Tuberculosis The bas
- Page 131 and 132: Resources and References Resources
- Page 133 and 134: Diagnosis of Latent Tuberculosis In
- Page 135 and 136: Forms All required and recommended
- Page 137 and 138: High-Risk Groups Certain factors id
- Page 139 and 140: Diagnosis of Latent Tuberculosis In
- Page 141 and 142: continually exposed to populations
- Page 143 and 144: Administration of the Tuberculin Sk
- Page 145 and 146: See “Two-Step Tuberculin Skin Tes
- Page 147 and 148: See “Live-Virus Vaccines” under
- Page 149 and 150: For more information on IGRAs and t
- Page 151 and 152: Table 5: TARGETED TESTING FOR LATEN
- Page 153 and 154: Resources and References Resources
- Page 155 and 156: Treatment of Latent Tuberculosis In
- Page 157 and 158: Policy Detailed information on the
- Page 159 and 160: Window period prophylaxis is treatm
Reporting Reactions<br />
The table below is intended for use by providers and public health nurses who perform<br />
case management services. Instruct the patient to report any side effects and adverse<br />
reactions listed in Table 6.<br />
If a patient reports an adverse reaction, the provider or public health nurse should alert<br />
the <strong>Alaska</strong> TB program by calling 907-269-8000.<br />
Table 6: REPORTING REACTIONS TO ANTITUBERCULOSIS MEDICATIONS 16<br />
Potentially Serious<br />
Adverse Reactions*<br />
Immediately report the following signs and symptoms<br />
or other abnormalities or unexpected events to the<br />
patient’s provider. These signs and symptoms<br />
suggest side effects, including hepatotoxicity:<br />
Jaundice<br />
Dark urine<br />
Vomiting<br />
Abdominal pain<br />
Fever<br />
Visual changes<br />
Marked clinical rash<br />
In consultation with the provider, instruct the patient<br />
to stop TB medications until evaluated by the<br />
provider.<br />
Less Severe<br />
Signs and Symptoms*<br />
Report the following signs and symptoms to the<br />
patient’s provider within 24 hours:<br />
Anorexia<br />
Nausea<br />
Malaise<br />
Peripheral neuropathy: tingling or burning sensation<br />
in hands or feet<br />
Rashes<br />
* These lists are not all-inclusive. Second-line drugs are not included. For a complete list, refer to the current guidelines for<br />
treatment <strong>of</strong> TB, “Treatment <strong>of</strong> <strong>Tuberculosis</strong>” (MMWR 2003;52[No. RR-11]), at<br />
http://www.cdc.gov/mmwr/PDF/rr/rr5211.pdf .<br />
Source: California Department <strong>of</strong> Health Services (CDHS)/California <strong>Tuberculosis</strong> Controllers Association (CTCA). TB<br />
case management—core components. CDHS/CTCA Joint Guidelines [CTCA Web site]. November 2011 Available at:<br />
http://www.ctca.org/fileLibrary/file_238.pdf Accessed October 20, 2012.<br />
ALASKA T U B E R C U L O S I S P R O G R A M M A N U A L Treatment <strong>of</strong> Tuber culosis Disease 6.20<br />
Revised November 2012