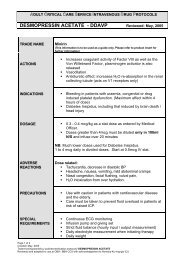
Stabilisation of an Endotracheal Tube for the Adult Intensive Care ...
Stabilisation of an Endotracheal Tube for the Adult Intensive Care ...
Stabilisation of an Endotracheal Tube for the Adult Intensive Care ...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
4. Taxonomy <strong>for</strong> level <strong>of</strong> evidence <strong>an</strong>d grade <strong>of</strong> recommendation<br />
Table 4: NHMRC Designations <strong>of</strong> levels <strong>of</strong> Evidence<br />
Level Intervention Numbers <strong>of</strong> Studies found<br />
I A systematic review <strong>of</strong> level II studies 1<br />
II A r<strong>an</strong>domised controlled trial<br />
III-1<br />
III-2<br />
III-3<br />
IV<br />
Table 5: NHMRC Grading <strong>of</strong> Evidence<br />
Component<br />
Volume <strong>of</strong><br />
evidence<br />
A pseudor<strong>an</strong>domised controlled trial<br />
(i.e. alternate allocation or some o<strong>the</strong>r method)<br />
A comparative study with concurrent controls:<br />
• Non-r<strong>an</strong>domised, experimental trial<br />
• Cohort study<br />
• Case-control study<br />
• Interrupted time series with a control group<br />
A comparative study without concurrent controls:<br />
• Historical control study<br />
• Two or more single arm study<br />
• Interrupted time series without a parallel control<br />
group<br />
Case series with ei<strong>the</strong>r post-test or pre-test/posttest<br />
outcomes<br />
A B C D<br />
Excellent Good Satisfactory Poor<br />
several level I or II<br />
studies with low risk<br />
<strong>of</strong> bias<br />
Consistency all studies consistent<br />
one or two level II<br />
studies with low risk <strong>of</strong><br />
bias or a SR/multiple<br />
level III studies with<br />
low risk <strong>of</strong> bias<br />
most studies<br />
consistent <strong>an</strong>d<br />
inconsistency may be<br />
explained<br />
level III studies with low risk<br />
<strong>of</strong> bias, or level I or II<br />
studies with moderate risk<br />
<strong>of</strong> bias<br />
some inconsistency<br />
reflecting genuine<br />
uncertainty around clinical<br />
question<br />
2<br />
24<br />
level IV studies, or level I<br />
to III studies with high risk<br />
<strong>of</strong> bias<br />
evidence is inconsistent<br />
Clinical impact very large subst<strong>an</strong>tial moderate slight or restricted<br />
Generalisability<br />
Applicability<br />
population/s studied<br />
in body <strong>of</strong> evidence<br />
are <strong>the</strong> same as <strong>the</strong><br />
target population <strong>for</strong><br />
<strong>the</strong> guideline<br />
directly applicable to<br />
Australi<strong>an</strong> healthcare<br />
context<br />
population/s studied in<br />
<strong>the</strong> body <strong>of</strong> evidence<br />
are similar to <strong>the</strong><br />
target population <strong>for</strong><br />
<strong>the</strong> guideline<br />
applicable to<br />
Australi<strong>an</strong> healthcare<br />
context with few<br />
caveats<br />
population/s studied in body<br />
<strong>of</strong> evidence different to<br />
target population <strong>for</strong><br />
guideline but it is clinically<br />
sensible to apply this<br />
evidence to target<br />
population<br />
probably applicable to<br />
Australi<strong>an</strong> healthcare<br />
context with some caveats<br />
population/s studied in<br />
body <strong>of</strong> evidence different<br />
to target population <strong>an</strong>d<br />
hard to judge whe<strong>the</strong>r it is<br />
sensible to generalise to<br />
target population<br />
not applicable to<br />
Australi<strong>an</strong> healthcare<br />
context