Tellurite And Fluorotellurite Glasses For Active And Passive
Tellurite And Fluorotellurite Glasses For Active And Passive Tellurite And Fluorotellurite Glasses For Active And Passive
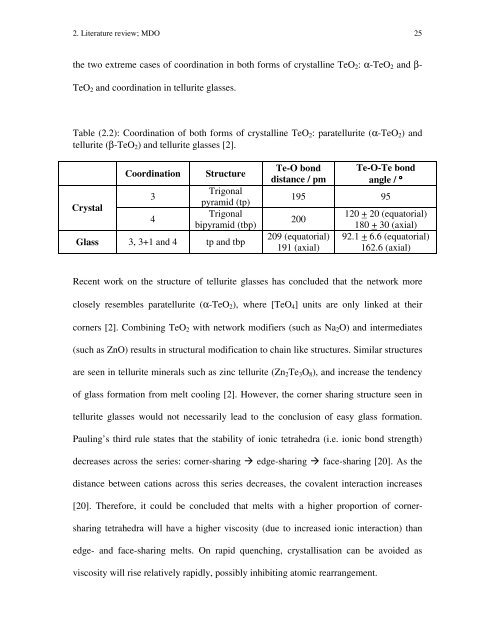
2. Literature review; MDO 25 the two extreme cases of coordination in both forms of crystalline TeO2: α-TeO2 and β- TeO2 and coordination in tellurite glasses. Table (2.2): Coordination of both forms of crystalline TeO2: paratellurite (α-TeO2) and tellurite (β-TeO2) and tellurite glasses [2]. Crystal Coordination Structure 3 4 Trigonal pyramid (tp) Trigonal bipyramid (tbp) Glass 3, 3+1 and 4 tp and tbp Te-O bond distance / pm Te-O-Te bond angle / ° 195 95 200 209 (equatorial) 191 (axial) 120 + 20 (equatorial) 180 + 30 (axial) 92.1 + 6.6 (equatorial) 162.6 (axial) Recent work on the structure of tellurite glasses has concluded that the network more closely resembles paratellurite (α-TeO2), where [TeO4] units are only linked at their corners [2]. Combining TeO2 with network modifiers (such as Na2O) and intermediates (such as ZnO) results in structural modification to chain like structures. Similar structures are seen in tellurite minerals such as zinc tellurite (Zn2Te3O8), and increase the tendency of glass formation from melt cooling [2]. However, the corner sharing structure seen in tellurite glasses would not necessarily lead to the conclusion of easy glass formation. Pauling’s third rule states that the stability of ionic tetrahedra (i.e. ionic bond strength) decreases across the series: corner-sharing edge-sharing face-sharing [20]. As the distance between cations across this series decreases, the covalent interaction increases [20]. Therefore, it could be concluded that melts with a higher proportion of corner- sharing tetrahedra will have a higher viscosity (due to increased ionic interaction) than edge- and face-sharing melts. On rapid quenching, crystallisation can be avoided as viscosity will rise relatively rapidly, possibly inhibiting atomic rearrangement.
2. Literature review; MDO 26 Champarnaud-Mesjard et al. [19, 21] have recently found evidence of γ- and δ-TeO2 phases in crystalline and glassy samples. γ-TeO2 was formed by heat treating binary TeO2 glasses which contain WO3, Nb2O5 or PbO (5-10 mol. %). The glasses were slowly heated to 440°C and held at this temperature for 60 hours. The crystal structure of this phase was determined by XRD to be orthorhombic, space group P212121. A band at 430 cm -1 in the Raman spectra of these glasses corresponded to a similar structural unit to γ- TeO2 [21]. δ-TeO2 is thought to be an intermediate phase between the crystalline and glassy state (an ‘antiglass’) [19]. This phase was formed by annealing TeO2-WO3 compositions (5-10 mol. %) for 25 hours at 340°C. δ-TeO2 was determined by XRD to be a metastable fluorite-related cubic structure, space group Fm 3 m [19]. 2.3.2.2. TeO2-ZnO The ZnO crystal structure is hexagonal, space group P63mc, analogous to wurtzite (ZnS). Oxygen anions are hexagonally close packed (hcp) with alternate tetrahedral voids filled with zinc cations [20]. Both zinc and oxygen are four coordinated to one another, therefore the structure can be thought of as interpenetrating hcp sublattices of Zn and O [20]. Kozhukharov studied the structure of 80TeO2-20ZnO mol. % glass with neutron diffraction [22]. This study showed that the structural units in the glass ([TeO4], [TeO3] and [ZnO6]) are similar to those in α-TeO2, and Zn2Te3O8, but predominantly the latter. The average coordination number of tellurium was found to be 3.35. Interatomic (Te-O,
- Page 1 and 2: TELLURITE AND FLUOROTELLURITE GLASS
- Page 3 and 4: Abstract Glasses systems based on T
- Page 5 and 6: Contents; MDO i Contents Contents i
- Page 7 and 8: Contents; MDO iii 6.1.2.1. Method a
- Page 9 and 10: Glossary; MDO Glossary aM = partial
- Page 11 and 12: Glossary; MDO λ = wavelength λn =
- Page 13 and 14: Glossary; MDO Ψ = complex dielectr
- Page 15 and 16: 1. Introduction; MDO 2 communicatio
- Page 17 and 18: 1. Introduction; MDO 4 Infrared tra
- Page 19 and 20: 1. Introduction; MDO 6 1.4. Thesis
- Page 21 and 22: 1. Introduction; MDO 8 [14] J. E. S
- Page 23 and 24: 2. Literature review; MDO 10 one of
- Page 25 and 26: 2. Literature review; MDO 12 superc
- Page 27 and 28: 2. Literature review; MDO 14 2.2.2.
- Page 29 and 30: 2. Literature review; MDO 16 phenom
- Page 31 and 32: 2. Literature review; MDO 18 tenden
- Page 33 and 34: 2. Literature review; MDO 20 crysta
- Page 35 and 36: 2. Literature review; MDO 22 the Za
- Page 37: 2. Literature review; MDO 24 2.3.2.
- Page 41 and 42: 2. Literature review; MDO 28 the ad
- Page 43 and 44: 2. Literature review; MDO 30 (a) (b
- Page 45 and 46: 2. Literature review; MDO 32 showed
- Page 47 and 48: 2. Literature review; MDO 34 absorp
- Page 49 and 50: 2. Literature review; MDO 36 sharp
- Page 51 and 52: 2. Literature review; MDO 38 near f
- Page 53 and 54: 2. Literature review; MDO 40 where
- Page 55 and 56: 2. Literature review; MDO 42 Transm
- Page 57 and 58: 2. Literature review; MDO 44 origin
- Page 59 and 60: 2. Literature review; MDO 46 4 α =
- Page 61 and 62: 2. Literature review; MDO 48 Bragli
- Page 63 and 64: 2. Literature review; MDO 50 It can
- Page 65 and 66: 2. Literature review; MDO 52 and su
- Page 67 and 68: 2. Literature review; MDO 54 be bro
- Page 69 and 70: 2. Literature review; MDO 56 2.5.2.
- Page 71 and 72: 2. Literature review; MDO 58 Table
- Page 73 and 74: 2. Literature review; MDO 60 2.5.2.
- Page 75 and 76: 2. Literature review; MDO 62 [20] J
- Page 77 and 78: 2. Literature review; MDO 64 [47] S
- Page 79 and 80: 3. Glass batching and melting; MDO
- Page 81 and 82: 3. Glass batching and melting; MDO
- Page 83 and 84: 3. Glass batching and melting; MDO
- Page 85 and 86: 3. Glass batching and melting; MDO
- Page 87 and 88: 3. Glass batching and melting; MDO
2. Literature review; MDO 25<br />
the two extreme cases of coordination in both forms of crystalline TeO2: α-TeO2 and β-<br />
TeO2 and coordination in tellurite glasses.<br />
Table (2.2): Coordination of both forms of crystalline TeO2: paratellurite (α-TeO2) and<br />
tellurite (β-TeO2) and tellurite glasses [2].<br />
Crystal<br />
Coordination Structure<br />
3<br />
4<br />
Trigonal<br />
pyramid (tp)<br />
Trigonal<br />
bipyramid (tbp)<br />
Glass 3, 3+1 and 4 tp and tbp<br />
Te-O bond<br />
distance / pm<br />
Te-O-Te bond<br />
angle / °<br />
195 95<br />
200<br />
209 (equatorial)<br />
191 (axial)<br />
120 + 20 (equatorial)<br />
180 + 30 (axial)<br />
92.1 + 6.6 (equatorial)<br />
162.6 (axial)<br />
Recent work on the structure of tellurite glasses has concluded that the network more<br />
closely resembles paratellurite (α-TeO2), where [TeO4] units are only linked at their<br />
corners [2]. Combining TeO2 with network modifiers (such as Na2O) and intermediates<br />
(such as ZnO) results in structural modification to chain like structures. Similar structures<br />
are seen in tellurite minerals such as zinc tellurite (Zn2Te3O8), and increase the tendency<br />
of glass formation from melt cooling [2]. However, the corner sharing structure seen in<br />
tellurite glasses would not necessarily lead to the conclusion of easy glass formation.<br />
Pauling’s third rule states that the stability of ionic tetrahedra (i.e. ionic bond strength)<br />
decreases across the series: corner-sharing edge-sharing face-sharing [20]. As the<br />
distance between cations across this series decreases, the covalent interaction increases<br />
[20]. Therefore, it could be concluded that melts with a higher proportion of corner-<br />
sharing tetrahedra will have a higher viscosity (due to increased ionic interaction) than<br />
edge- and face-sharing melts. On rapid quenching, crystallisation can be avoided as<br />
viscosity will rise relatively rapidly, possibly inhibiting atomic rearrangement.