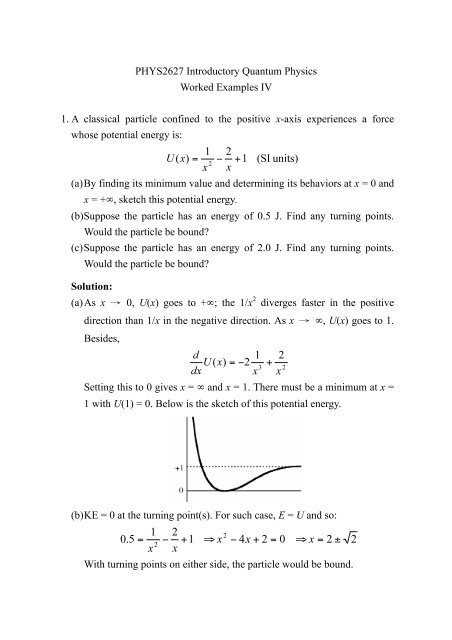
PHYS2627 Introductory Quantum Physics Worked Examples IV 1. A ...
PHYS2627 Introductory Quantum Physics Worked Examples IV 1. A ...
PHYS2627 Introductory Quantum Physics Worked Examples IV 1. A ...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
(c) KE = 0 at the turning point(s). For such case, E = U and so:<br />
The only positive root is . There will be a turning point on<br />
only one side. Thus the particle would not be bound.
2. A wave has the form<br />
when x < 0. For x > 0, the wavelength is λ/2. By applying continuity<br />
conditions at x = 0, find the amplitude (in terms of A) and phase of the<br />
wave in the region x > 0. Sketch the wave, showing both x < 0 and x > 0.<br />
Solution:<br />
For x < 0,<br />
For x > 0,<br />
At , ⇒<br />
At , ⇒<br />
Dividing the second of these equations by the first, we obtain<br />
Thus<br />
or
3. The following figure shows the wave function of an electron in a rigid box.<br />
The electron energy is 6.0 eV. How long is this box?<br />
Solution:<br />
The energy levels for a particle in a rigid box are<br />
The wave function shown in the figure corresponds to the n = 3 quantum<br />
state. Thus,
4. Consider a particle moving in a one-dimensional box with walls at x = -L/2<br />
and x = L/2.<br />
(a) Write down the wave functions and probability densities for the states n<br />
= 1, n = 2, and n = 3.<br />
(b) Sketch the wave functions and probability densities. (Hint: Make an<br />
analogy to the case of a particle in a box with walls at x = 0 and x = L.)<br />
Solution:<br />
(a) In the present case, the box is displaced from (0, L) by . Accordingly,<br />
we may obtain the wave functions of our box by replacing x with<br />
L. Using<br />
we get for<br />
in the wave functions of a 1-D box with walls at x = 0 and x =<br />
(b) Below are the wave functions and probability densities for n = 1, 2 and<br />
3.<br />
€<br />
|ψ 1 (x) | 2
5. An electron is trapped at a defect in a crystal. The defect may be modeled<br />
as a one dimensional, rigid-walled box of width <strong>1.</strong>00 nm.<br />
(a) Sketch the wave functions and probability densities for the n = 1 and n =<br />
2 states.<br />
(b) For the n = 1 state, find the probability of finding the electron between<br />
x1 = 0.15 nm and x2 = 0.35 nm, where x = 0 is the left side of the box.<br />
(c) Repeat part (b) for the n = 2 state.<br />
(d) Calculate the energies in electron volts for the n = 1 and n = 2 states.<br />
Solution:<br />
(a) The wave functions and probability densities of the n = 1 and n = 2<br />
states are shown below:<br />
(b) Expressing all lengths in units of 10 -10 m, we find<br />
Using the formula , we obtain<br />
(c) Similar to part (b),
(d) Using , we find and .<br />
6. Sketch the n = 8 wave function for the potential energy shown in following<br />
figure.<br />
Solution:<br />
There are three factors to consider. First, the de Broglie wavelength<br />
increases as the particle’s speed and kinetic energy decreases. Thus, the<br />
spacing between the nodes of y(x) increases in regions where U is larger.<br />
Second, a particle is more likely to be found where it is moving the slowest.<br />
Thus, the amplitude of y(x) increases in regions where U is larger. Third,<br />
for n = 8 there will be 8 antinodes to place.<br />
(Note that the wavelength should keep constant in each region of constant<br />
potential.)
7. In most metals, the atomic ions form a regular arrangement called a crystal<br />
lattice. The conduction electrons in the sea of electrons move through this<br />
lattice. Below figure is a one dimensional model of a crystal lattice. The<br />
ions have mass m, charge e, and equilibrium separation b.<br />
(a) Suppose the middle charge is displaced a very small distance (x
(b) The force in part (a) is a linear restoring force of the form F = –kx. This<br />
is Hooke’s law with, in this case, “spring constant” .<br />
The potential energy of the "spring" is<br />
Thus the angular frequency of vibration is<br />
The ground state energy is<br />
Likewise,<br />
.<br />
(c) ΔE between two adjacent levels from part (b) is E2 – E1 = E3 – E2 = E4 –<br />
E3 = 0.0182 eV. The wavelength of a photon with this energy is<br />
The wavelength is in the far infrared region.
8. The wave function<br />
describes a state of the quantum oscillator provided that the constant α is<br />
chosen appropriately.<br />
(a) Using Schrödinger's equation, obtain an expression for α in terms of the<br />
oscillator mass m and the classical frequency of vibration ω. What is the<br />
energy of this state?<br />
(b) What is the quantum number of this state?<br />
(c) Find the normalization constant C.<br />
(Useful formula: for a > 0.)<br />
Solution:<br />
(a) After rearrangement, the Schrödinger equation is<br />
where for a quantum oscillator.<br />
Differentiating gives<br />
and<br />
Therefore, for ψ(x) to be a solution requires<br />
Equating coefficients of like terms gives<br />
and . Thus,<br />
and .
(b) The quantum number is equal to 1 since the energy of the nth state of a<br />
quantum harmonic oscillator is .<br />
(c) The normalization integral is<br />
where the second step follows from the symmetry of the integrand about<br />
x = 0. Identifying a with 2α in the integral<br />
gives