Download (8Mb) - Etheses - Saurashtra University
Download (8Mb) - Etheses - Saurashtra University
Download (8Mb) - Etheses - Saurashtra University
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
No.<br />
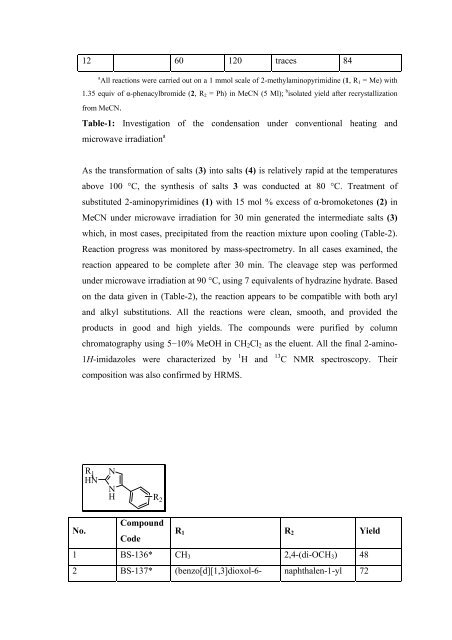
12 60 120 traces 84<br />
a<br />
All reactions were carried out on a 1 mmol scale of 2-methylaminopyrimidine (1, R1 = Me) with<br />
1.35 equiv of α-phenacylbromide (2, R2 = Ph) in MeCN (5 Ml); b isolated yield after recrystallization<br />
from MeCN.<br />
Table-1: Investigation of the condensation under conventional heating and<br />
microwave irradiation a<br />
As the transformation of salts (3) into salts (4) is relatively rapid at the temperatures<br />
above 100 °C, the synthesis of salts 3 was conducted at 80 °C. Treatment of<br />
substituted 2-aminopyrimidines (1) with 15 mol % excess of α-bromoketones (2) in<br />
MeCN under microwave irradiation for 30 min generated the intermediate salts (3)<br />
which, in most cases, precipitated from the reaction mixture upon cooling (Table-2).<br />
Reaction progress was monitored by mass-spectrometry. In all cases examined, the<br />
reaction appeared to be complete after 30 min. The cleavage step was performed<br />
under microwave irradiation at 90 °C, using 7 equivalents of hydrazine hydrate. Based<br />
on the data given in (Table-2), the reaction appears to be compatible with both aryl<br />
and alkyl substitutions. All the reactions were clean, smooth, and provided the<br />
products in good and high yields. The compounds were purified by column<br />
chromatography using 5−10% MeOH in CH2Cl2 as the eluent. All the final 2-amino-<br />
1H-imidazoles were characterized by 1 H and 13 C NMR spectroscopy. Their<br />
composition was also confirmed by HRMS.<br />
R1 HN<br />
N<br />
N<br />
H<br />
R 2<br />
Compound<br />
Code<br />
R1 R2 Yield<br />
1 BS-136* CH3 2,4-(di-OCH3) 48<br />
2 BS-137* (benzo[d][1,3]dioxol-6- naphthalen-1-yl 72