the coking properties of coal at elevated pressures. - Argonne ...
the coking properties of coal at elevated pressures. - Argonne ... the coking properties of coal at elevated pressures. - Argonne ...
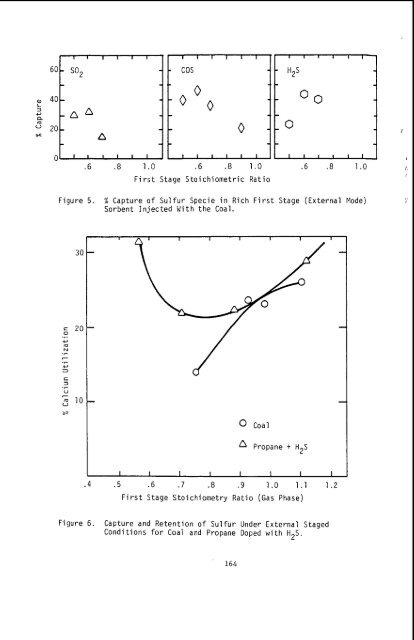
60- a, 40- 3 +J Q m as 20- 0 I 1 1 1 1 1 - SO2 - - - -A A - - n - 1 1 1 1 1 1 ' Figure 5. % Capture of Sulfur Specie in Rich First Stage (External Mode) Sorbent Injected With the Coal. 30 i= 20 0 .r +J m N .C 7 .r +J 1 E 2 .C u 7 2 10 %? v OC a1 Propane + H ~ S I I I I I I I I .4 .5 .6 .7 .8 .9 1.0 1.1 1.2 First Stage Stoichiometry Ratio (Gas Phase) Figure 6. Capture and Retention of Sulfur Under External Staged Conditions for Coai and Propane Doped with H2S. 164
under which conditions sulfur species generated during the combustion of pulverized coal can be captured and retained by calcium containing sorbents. Two series of experiments were carried out: one in which any capture would take place primarily under oxidizing conditions and the other in which significant residence times in the rich zone would allow capture under reducing conditions. Under oxidizing conditions the thermal environment experienced by the sorbent particle appears to be the dominant parameter controlling sulfur capture. This is probably because of deadburning. If a sorbent particle's temperature exceeds a certain limit (which depends on the particular sorbent) the sorbent deadburns and loses its reactivity (4). The processes controlling capture and retention when the sorbent is maintained under reducing conditions for a prolonged time are more complex. The principle gas phase sulfur specie are HzS, SO2 and COS and, even though the sulfur species are absorbed the possibility that the sulfide will decompose during burnout exists. The data presented in Figure 6 shows a significant difference between the behavior of coal and propane doped with H2S. This difference can be attributed to: - With coal part of the fuel remains in the solid phase and for a given input stoichiometry the gas phase stoichiometry in the reducing zone is higher than with gas. Reference to Figure 1 indicates that the stability of calcium sulfide is strongly dependent upon stoichiometry ratio; - With coal up to 50 percent of the sulfur remains in the solid phase under rich conditions thus the gas phase concentration is lower than the corres- ponding concentration with propane as the fuel; - The conditions during burnout in the second stage will be different for the solid and gaseous fuels and this could affect retention of the sulfur during burnout. These tests indicate that there is the potential to remove greater than 50 percent of the input sulfur with Ca/S molar ratios of two when coal is burned under low NOx conditions. Further work is necessary to insure that the controlling conditions can be achieved in practical combustors and that the sorbent injection does not adversely impact combustor performance. References 1. 2. 3. 4. 5. 6. Gartrell, F.F., "Full Scale Desulfurization of Stack Gas by Dry Limestone Injection", EPA-650/2-73-019 a, b, c, 1973. Zallen, D.M., Gershman, R., Heap, M.P., Nurick, W.H., "The Generalization of Low Emission Coal Burner Technology", Proceedings, Third Stationary Source Combustion Symposium, EPA-600/7-70-050 b, 1979. Flament, G., "The Simultaneous Reduction of NOx and SO2 in Coal Flames by Direct Injection of Sorbents in a Staged Mixing Burner", IFRF doc. no. G 19/a/10, 1981. Coutant, R.W., et al., "Investigation of the Reactivity of Limestone and Dolomite for Capturing SO2 from Flue Gas", Batelle Memorial Institute, Final Report, EPA Contract PH-86-67-115, 1970. Borgwardt, R.H., "Kinetic of the Reaction of SO2 with Calcined Limestone", NAPCA, Vol. 4, p. 59, 1970. Borgwardt, R.H., Presentation at the EPA SPO Contractors Meeting, Raleigh, North Carolina, October 1981. 165
- Page 113 and 114: Preparing process flow diagrams and
- Page 115 and 116: \ \ i 1. 2,000 4,000 6,000 8,000 10
- Page 117 and 118: (JMS-OlBM-2). The mass spectra were
- Page 119 and 120: I I Fairbridge, R.W. (ed.) 1972, En
- Page 121 and 122: v- W V z d 3 z m 4 ? P m W c3 W m N
- Page 123 and 124: e. \ I , W 2 m 4 t- CI 99-79 noooo
- Page 125 and 126: L Table I: Compositions of High Tem
- Page 127 and 128: \ " \ I For all three coals, the pr
- Page 129 and 130: 4 , 1024 1024 3a 3b J Mineral Parti
- Page 131 and 132: The Determination of Mineral Distri
- Page 133 and 134: pyrite crystals in framboids locate
- Page 135 and 136: FIG. 1. TEM MICROGRAPH OF A HIGH VO
- Page 137 and 138: \ \ FIG. 5. SEM MICROGRAPH SHOWING
- Page 139 and 140: \ , species. In fact, combustion ex
- Page 141 and 142: 1, It is possible to make a reasona
- Page 143 and 144: \ i SURFACE AND BULK ENRICHMENT OF
- Page 145 and 146: I Coal Ash Sintering Model and the
- Page 147 and 148: 5 carried out such sintering measur
- Page 149 and 150: I this ash and other bituminous coa
- Page 151 and 152: forming propensity. However, none o
- Page 153 and 154: t I 153
- Page 155 and 156: CENTRAL ELECTRICIN RESEARCH LABORAT
- Page 157 and 158: 157 18 I I a I
- Page 159 and 160: temperature, sulfur species concent
- Page 161 and 162: The facility is fired with coal usi
- Page 163: Tests have been carried out to dete
- Page 167 and 168: I TITANIUM L Corn AS A TRACER FOR D
- Page 169 and 170: E 6 i i h \ 1 1 \ on the XRF is cau
- Page 171 and 172: \ ? CONCLUSIONS Titanium can accura
- Page 173 and 174: NY) m * oa
- Page 175 and 176: GFETC constructed a 0.2 square mete
- Page 177 and 178: \: Stage 3. Sulfated ash-cemented a
- Page 179 and 180: I FIG. 1. Initial ash coating on qu
- Page 181 and 182: FIG 9. Limestone bed material alter
- Page 183 and 184: (4) Since the operating temperature
- Page 185 and 186: I I maximum particle diameter leavi
- Page 187 and 188: ! suspension chambers and cyclone f
- Page 189 and 190: Pilot Plant of a Coal Fired Fluidiz
- Page 191 and 192: After the fiscal year 1982, the fol
- Page 193 and 194: MBC CBC Fig. 1 Boiler Structure 193
- Page 195 and 196: \ i Table 2. Coal Properties Planne
- Page 197 and 198: -2- more than 3 years, since 1977.
- Page 199 and 200: , I B. 2 which temporarily raised t
- Page 201 and 202: -6- abrasion rate at the bottom of
- Page 203 and 204: \if F3.3 - Particulate Circulation
- Page 205 and 206: \' I/O. analog 1/13. industrial I/O
- Page 207 and 208: ACQUISITION DATA UNIT < I L- J (T)
- Page 209 and 210: SAMPLING SYSTEM FOR FLUIDIZED BED A
- Page 211 and 212: GAS SAMPLING WITH ORIGINAL PROBE-FI
- Page 213 and 214: Remove the quartz tube (gas sample
60-<br />
a, 40-<br />
3<br />
+J Q<br />
m<br />
as<br />
20-<br />
0<br />
I 1 1 1 1 1 -<br />
SO2 -<br />
- -<br />
-A A<br />
-<br />
- n -<br />
1 1 1 1 1 1 '<br />
Figure 5. % Capture <strong>of</strong> Sulfur Specie in Rich First Stage (External Mode)<br />
Sorbent Injected With <strong>the</strong> Coal.<br />
30<br />
i= 20<br />
0<br />
.r<br />
+J<br />
m N<br />
.C<br />
7 .r<br />
+J<br />
1<br />
E<br />
2<br />
.C<br />
u<br />
7<br />
2 10<br />
%?<br />
v<br />
OC a1<br />
Propane + H ~ S<br />
I I I I I I I I<br />
.4 .5 .6 .7 .8 .9 1.0 1.1 1.2<br />
First Stage Stoichiometry R<strong>at</strong>io (Gas Phase)<br />
Figure 6. Capture and Retention <strong>of</strong> Sulfur Under External Staged<br />
Conditions for Coai and Propane Doped with H2S.<br />
164