liquefaction pathways of bituminous subbituminous coals andtheir
liquefaction pathways of bituminous subbituminous coals andtheir liquefaction pathways of bituminous subbituminous coals andtheir
1 I HA0 and naohthalene extractions Table 2 indicates that the effect of the chlorobenzene treatment upon HA0 extraction of Point of Ayr coal is similar to that for tetralin with the yield of DCM-solubles increasing by cu 10% daf coal. The fact that HA0 is largely in the liquid rather than the vapour phase at 400°C suggests that mass transfer limitations on controlling the initial rate of conversion are still severe. The oil yields obtained with non-hydrogendonor aromatic compounds, such as naphthalene are obviously much lower than with effective donor solvents and this is reflected in the DCM-soluble yields in Table 2. However, as discussed earlier, aromatic compounds can give high conversions to pyridindquinoline-solubles (CU 80% daf basis) for some bituminous coals (3) probably with the aid of hydrogen shuttling or radical mediated hydrogen radical transfer in the case of a number of 4 and 5 ring systems, such as pyrene. Table 2 indicates that the chlorobenzene treatment has reduced the yield of pyridine-insolubles for Point of Ayr coal with naphthalene but has had little effect for the lower rank UKbituminous coal, Bentinck. It was reported previously (3) for a different sample of Point of Ayr coal that THF extraction lead to significant improvements in primary conversions to pyridine-solubles with both naphthalene and pyrene. In the case of Bentinck coal, it could well be that the primary conversion is limited by the lack of available hydrogen rather than by solvent accessibility. General discussion This investigation has reinforced other recent work (1-7) which has indicated that pre- treatments can greatly enhance liquefaction yields, particularly in relatively low-severity regimes. However, much of the other work was concerned with pre-swelling coals in polar solvents where the dominant effect is undoubtedly the disruption of hydrogen bonding. The increases in oil yield obtained here of 20-25% upon chlorobenzene treatment for the two bituminous coals of lowest rank (Pittsburgh No.8 and Bentinck) are somewhat greater than that of 14% reported by Joseph (3) for tetralin extraction (with a hydrogen over-pressure) of an Illinois N0.6 coal pre-treated with tetrabutlyammonium hydroxide (TBAH). Increases of only 5% were obtained for THF and methanol.but, nonetheless, pre-swelling with both these weakly-basic solvents increased the overall conversions to THF-solubles more than with TBAH. However, their boiling points are considerably lower than that of 132°C for chlorobenzene and, although some disruption df the hydrogen-bonding network in coals would undoubtedly have occurred as the coal swells to a significant extent in these polar solvents, it is uncertain whether the glass transition temperature would have been lowered sufficiently to allow the coal to adopt a lower energy configuration as with chlorobenzene. The nature of the conformational changes have yet to be fully . elucidated and are currently being investigated in our laboratory by a combination of DSC, surface area measurements, broadline IH NMR and electron microscopy. However, they are clearly different to those induced by polar solvents and could well involve the disruption of non-covalent bonding between aromatic moieties. There would appear to be considerable scope for improving conversions with process- derived solvents through pretreatments. Although it is clearly impractical to consider the chlorobenzene treatment in terms of a liquefaction process, the fact that it has been shown that the conformational changes can be brought about with a relatively short treatment time (3 hours, Table 1) provides a basis for exploiting such treatments above the glass transition temperatures of bituminous coals with actual process solvents. 568
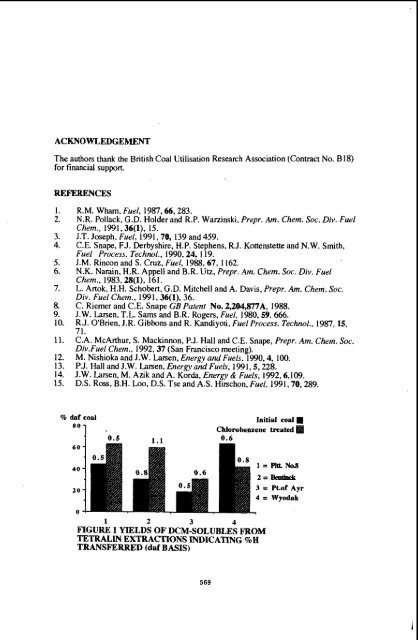
ACKNOWLEDGEMENT The authors thank the British Coal Utilisation Research Association (Contract No. B18) for financial support. REFERENCES 1. R.M. Wham, Fuel, 1987,66,283. 2. N.R. Pollack, G.D. Holder and R.P. Waninski, Prepr. Am. Chem. Soc. Div. Fuel Chem., 1991,36(1), 15. 3. J.T. Joseph, Fuel, 1991,70, 139 and 459. 4. C.E. Snape, FJ. Derbyshire, H.P. Stephens, R.J. Kottenstetteand N.W. Smith, Fuel Process. Technol., 1990,24, 119. 5. J.M. Rincon and S. Cruz, Fuel, 1988,67, I 162. 6. N.K. Narain. H.R. Appell and B.R. Utz, Prepr. Am. Chem. Soc. Div. Fuel Chem., 1983,28(1), 161. 7. L. Artok, H.H. Schobert, G.D. Mitchell and A. Davis, Prepr. Am. Chem. Soc. Div. Fuel Chem., 1991,36(1), 36. 8. C. Riemer and C.E. Snape CB Patent No. 2,204,877A, 1988. 9. J. W. Larsen, T.L. Sams and B.R. Rogers, Fuel, 1980,59.666. 10. RJ. OBrien, J.R. Gibbons and R. Kandiyoti, Fuel Process. Technol., 1987,15, 71. 11. C.A. McArthur, S. Mackinnon, P.J. Hall and C.E. Snape, Prepr. Am. Chetn. Soc. Div.Fue1 Chem.. 1992,37 (San Francisco meeting). 12. M. Nishioka and J.W. Larsen, Energy and Fuels. 1990,4, 100. 13. PJ. Hall and J.W. Larsen, Energy and Fuels, 1991,5,228. 14. J.W. Larsen, M. Azik and A. Korda, Energy & Fuels, 1992,6,109. 15. D.S. Ross, B.H. Loo, D.S. Tse and A.S. Hirschon, Fuel, 1991,70,289. % daf coal 1 60 '0.5 Initial coal Chlorobenzene treated 0.6 40 20 0 1 2 3 4 FIGURE 1 YIELDS OF DCM-SOLUBLES FROM TETRALIN EXTRACTIONS INDICATING %H TRANSFERRED (daf BASIS) 569
- Page 43 and 44: Nominal 2 Table 1. Concentration of
- Page 45 and 46: - iF m 1.6 1.4 1.2 - - - 1- 0 0.8 -
- Page 47 and 48: RESULTS AND DISCUSSION Swelling of
- Page 49 and 50: . . % . . 9 'HF 0 0 0 *. . 0 . . 0
- Page 51 and 52: 1.50 KQ 1.00 0.50 I / ' 02 525 I 1
- Page 53 and 54: hydrogen atoms. The hydrogen atoms
- Page 55 and 56: D to generate more D atoms. It is r
- Page 57 and 58: 12. a. Poutsma, M. L.; Dyer, C. W.
- Page 59 and 60: Figure 3. Minimum Steps to Explin D
- Page 61 and 62: Apoaratus and Procedure Microflow R
- Page 63 and 64: Model ComDound Test Figure 5 shows
- Page 65 and 66: Figure 1. High resolution gas chrom
- Page 67 and 68: Figure 5. Product distribution for
- Page 69 and 70: THQ at somewhat higher temperatures
- Page 71 and 72: areas of the particles and the SEM
- Page 73 and 74: Experimental Catalyst Precursors an
- Page 75 and 76: impregnating solvent. Table 3 shows
- Page 77 and 78: of MoCo-TC2 at the level of 0.5 wt%
- Page 79 and 80: Table 4. Effect of Temperature Prog
- Page 81 and 82: In the past, chemical treatments in
- Page 83 and 84: The effect of Corn20 preaatment on
- Page 85 and 86: Reaction Time Figure 1 - Schematic
- Page 87 and 88: DISSOLUTION OF THE ARGONNE PREMIUM
- Page 89 and 90: A much more def~tive trend is seen
- Page 91 and 92: EFFECT OF CHLOROBENZENE TREATMENT O
- Page 93: same conditions and an extraction t
- Page 97 and 98: THE STRUCTURAL &=RATION OF HUMINlTE
- Page 99 and 100: to be originally derived from demet
- Page 101 and 102: 145 30 I --/---Jh I , , I I , 250 2
- Page 103 and 104: THE EFFECTS OF MOISTURE AND CATIONS
- Page 105 and 106: 1 for the Zap lignite. These result
- Page 107 and 108: content of the samples ion-exchange
- Page 109 and 110: Table 1. F'yrolysis Results of Vacu
- Page 111 and 112: 585 I
- Page 113 and 114: EFFECT OF TEMPERATURE, SAMPLE SI2E
- Page 115 and 116: of some of the thermobalance runs.
- Page 117 and 118: 2. The mechanism of drying is a uni
- Page 119 and 120: Influence of Drying and Oxidation 0
- Page 121 and 122: "c gives W conversion mpared to the
- Page 123 and 124: Table 1. Products dismbutions (dmmf
- Page 125 and 126: - 50 45 40 E 35 2 30 E T) 25 ap 20
- Page 127 and 128: Influence of Drying and Oxidation o
- Page 129 and 130: FTIR . . of the L m To investigate
- Page 131 and 132: CONCLUSIONS The characexizntion of
- Page 133 and 134: A b S 0 r b a n C e A b s 0 r b a n
- Page 135 and 136: An NMR Investigation of the Effd of
- Page 137 and 138: for determining the area of the pea
- Page 139 and 140: of the ronl roniponcnte nnd (2) the
- Page 141 and 142: 2w 180 160 9 140 120 P loo f 80 P O
- Page 143 and 144: 25 I 20 ' + 0 Drying lime, hours Fi
ACKNOWLEDGEMENT<br />
The authors thank the British Coal Utilisation Research Association (Contract No. B18)<br />
for financial support.<br />
REFERENCES<br />
1. R.M. Wham, Fuel, 1987,66,283.<br />
2. N.R. Pollack, G.D. Holder and R.P. Waninski, Prepr. Am. Chem. Soc. Div. Fuel<br />
Chem., 1991,36(1), 15.<br />
3. J.T. Joseph, Fuel, 1991,70, 139 and 459.<br />
4. C.E. Snape, FJ. Derbyshire, H.P. Stephens, R.J. Kottenstetteand N.W. Smith,<br />
Fuel Process. Technol., 1990,24, 119.<br />
5. J.M. Rincon and S. Cruz, Fuel, 1988,67, I 162.<br />
6. N.K. Narain. H.R. Appell and B.R. Utz, Prepr. Am. Chem. Soc. Div. Fuel<br />
Chem., 1983,28(1), 161.<br />
7. L. Artok, H.H. Schobert, G.D. Mitchell and A. Davis, Prepr. Am. Chem. Soc.<br />
Div. Fuel Chem., 1991,36(1), 36.<br />
8. C. Riemer and C.E. Snape CB Patent No. 2,204,877A, 1988.<br />
9. J. W. Larsen, T.L. Sams and B.R. Rogers, Fuel, 1980,59.666.<br />
10. RJ. OBrien, J.R. Gibbons and R. Kandiyoti, Fuel Process. Technol., 1987,15,<br />
71.<br />
11. C.A. McArthur, S. Mackinnon, P.J. Hall and C.E. Snape, Prepr. Am. Chetn. Soc.<br />
Div.Fue1 Chem.. 1992,37 (San Francisco meeting).<br />
12. M. Nishioka and J.W. Larsen, Energy and Fuels. 1990,4, 100.<br />
13. PJ. Hall and J.W. Larsen, Energy and Fuels, 1991,5,228.<br />
14. J.W. Larsen, M. Azik and A. Korda, Energy & Fuels, 1992,6,109.<br />
15. D.S. Ross, B.H. Loo, D.S. Tse and A.S. Hirschon, Fuel, 1991,70,289.<br />
% daf coal<br />
1 60<br />
'0.5<br />
Initial coal<br />
Chlorobenzene treated<br />
0.6<br />
40<br />
20<br />
0<br />
1 2 3 4<br />
FIGURE 1 YIELDS OF DCM-SOLUBLES FROM<br />
TETRALIN EXTRACTIONS INDICATING %H<br />
TRANSFERRED (daf BASIS)<br />
569