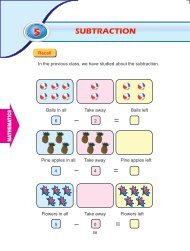
BOTANY Higher Secondary Second Year - Textbooks Online
BOTANY Higher Secondary Second Year - Textbooks Online
BOTANY Higher Secondary Second Year - Textbooks Online
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
Mechanism of enzyme action<br />
In a biochemical reaction, there is an energy barrier between the<br />
reactants and the products. Only those molecules which possess a certain<br />
amount of excess energy above the average energy of normal molecules<br />
are able to react to form products. This excess energy which a normal<br />
molecule must aquire in order to react is known as energy of activation<br />
(Ea). This energy of activation determines the rate of reaction. <strong>Higher</strong> the<br />
value of Ea, lower is the rate of reaction and greater stability. At higher<br />
ProductsPotential energy<br />
Reactants<br />
Energy of<br />
activation<br />
without<br />
enzyme<br />
Energy of activation<br />
with enzyme<br />
Products<br />
Reaction co-ordinates<br />
Fig. 5.4 Mechanism of enzyme action<br />
temperature, the rate<br />
of chemical reaction<br />
becomes faster, because<br />
increased temperature brings<br />
about increased number of<br />
activated molecules. But in<br />
the case of enzyme catalyzed<br />
reaction, the rate of reaction<br />
is optimum at normal body<br />
temperature. Because all the<br />
molecules either energy-rich<br />
or energy-poor combine with<br />
the active site of the enzyme<br />
to form enzyme substrate complex. The latter breaks into enzyme and<br />
product. Thus, the enzyme acts by lowering the energy of activation of<br />
the reactions i.e. reducing the energy barrier and increases the rate of<br />
reaction.<br />
Self evalution<br />
I . Choose and write the correct options.<br />
1. The term enzyme was coined by<br />
a. Kuhne b. Fischer<br />
c. Buchner d. Koshland<br />
2. The lock and key theory of enzyme action was proposed by<br />
a. Kuhne b. Fischer<br />
c. Buchner d. Koshland<br />
3. An example for transferase is<br />
a. transaminase b. pyruvic carboxylase<br />
c. histidine decarboxylase d. G-3-P dehydrogenase<br />
172