Desmopressin - Intensive Care & Coordination Monitoring Unit
Desmopressin - Intensive Care & Coordination Monitoring Unit
Desmopressin - Intensive Care & Coordination Monitoring Unit
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
Liverpool Health Service Drug Administration Protocol First Issued February 2002<br />
<strong>Intensive</strong> <strong>Care</strong> <strong>Unit</strong><br />
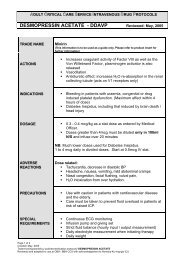
Contraindications<br />
Type IIB von Willebrand's disease.<br />
Habitual and psychogenic polydipsia.<br />
Cardiac insufficiency and other conditions requiring treatment with diuretic agents.<br />
Hypersensitivity to the preservative.<br />
<strong>Desmopressin</strong> is ineffective for the treatment of nephrogenic diabetes insipidus.<br />
Precautions<br />
Overhydration, especially with children, the elderly, when used with concurrent fluid replacement.<br />
Patients with a history of cardiac failure and when used to test renal concentrating ability.<br />
Excessive water intake or chronic use of desmopressin can produce hyponatraemia with<br />
associated effects.<br />
When desmopressin is used for diagnostic purposes, the fluid intake must be limited and not<br />
exceed 0.5 litres from one hour before until eight hours after administration.<br />
<strong>Desmopressin</strong> should not be administered to dehydrated or overhydrated patients until water<br />
balance has been adequately restored.<br />
In haemophilia, where high doses are given, extreme care must be paid to the water balance.<br />
Fluid intake should be restricted as much as possible and the patient should be weighed<br />
regularly.<br />
Not for intransal administration if rhinorrhoea or local infection exists.<br />
Use with caution in patients with cystic fibrosis because of impaired water handling and increased<br />
risk of hyponatraemia.<br />
Use with caution in patients at risk of increased intracranial pressure secondary to fluid retention.<br />
Significant Interactions<br />
Tricyclic antidepressants, chlorpromazine and carbamazepine may cause an additive antidiuretic<br />
effect and increase the risk of water retention.<br />
Indomethacin may augment the magnitude but not the duration of the response to desmopressin.<br />
Glibenclamide inhibits the antidiuretic effect of desmopressin.<br />
Clofibrate has potentiated and prolonged the effects of desmopressin.<br />
Adverse Effects<br />
Tachycardia, fall in diastolic blood pressure by 10 to 20% with large IV doses.<br />
Hypertension.<br />
Headache, nausea, mild abdominal cramp, vomiting.<br />
Nasal congestion, facial flushing, vulval pain.<br />
Water intoxication from overhydration, hyponatraemia.<br />
Presentation<br />
Intranasal solution, 100 micrograms/mL, 2.5mL dropper bottle plus rhinyle (Minirin).<br />
Injection, 4 micrograms/mL in 1mL ampoule (DDAVP).<br />
Injection, 15 micrograms/mL in 1mL ampoule (Octostim) for intravenous use only.<br />
Reviewed: September 2004 Authors: M. Edgtton-Winn Page 2 of 2<br />
Review Date: September 2005