The Benzoin Condensation
The Benzoin Condensation
The Benzoin Condensation
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
Although the benzoin condensation proceeds rapidly and produces benzoin in high yield when<br />
catalyzed by cyanide, we will not perform the reaction with this catalyst. Cyanide is extremely<br />
toxic and accidental exposure to even small amounts can have serious consequences. Cyanide is<br />
not readily absorbed through the skin, but any small cut or break in the skin can lead to accidental<br />
exposure. In solid form, it may also be inhaled in the form of dust or small particles.<br />
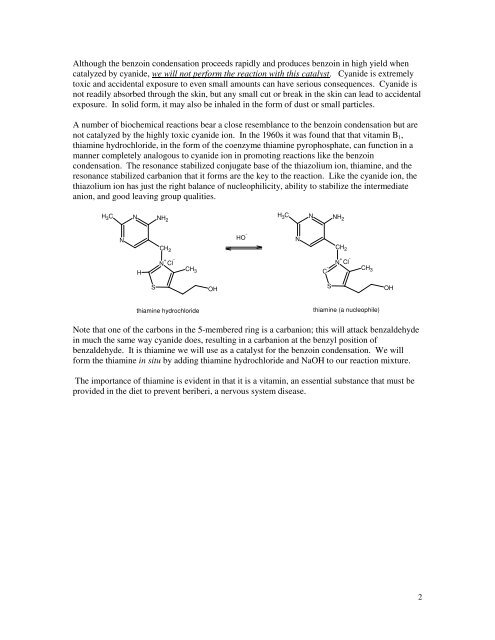
A number of biochemical reactions bear a close resemblance to the benzoin condensation but are<br />
not catalyzed by the highly toxic cyanide ion. In the 1960s it was found that that vitamin B1,<br />
thiamine hydrochloride, in the form of the coenzyme thiamine pyrophosphate, can function in a<br />
manner completely analogous to cyanide ion in promoting reactions like the benzoin<br />
condensation. <strong>The</strong> resonance stabilized conjugate base of the thiazolium ion, thiamine, and the<br />
resonance stabilized carbanion that it forms are the key to the reaction. Like the cyanide ion, the<br />
thiazolium ion has just the right balance of nucleophilicity, ability to stabilize the intermediate<br />
anion, and good leaving group qualities.<br />
H3C N NH2<br />
N<br />
H<br />
S<br />
CH 2<br />
N +<br />
Cl -<br />
CH 3<br />
OH<br />
HO -<br />
H3C N NH2<br />
N<br />
C -<br />
S<br />
CH 2<br />
N +<br />
Cl -<br />
thiamine hydrochloride thiamine (a nucleophile)<br />
Note that one of the carbons in the 5-membered ring is a carbanion; this will attack benzaldehyde<br />
in much the same way cyanide does, resulting in a carbanion at the benzyl position of<br />
benzaldehyde. It is thiamine we will use as a catalyst for the benzoin condensation. We will<br />
form the thiamine in situ by adding thiamine hydrochloride and NaOH to our reaction mixture.<br />
<strong>The</strong> importance of thiamine is evident in that it is a vitamin, an essential substance that must be<br />
provided in the diet to prevent beriberi, a nervous system disease.<br />
CH 3<br />
OH<br />
2