The Benzoin Condensation
The Benzoin Condensation
The Benzoin Condensation
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
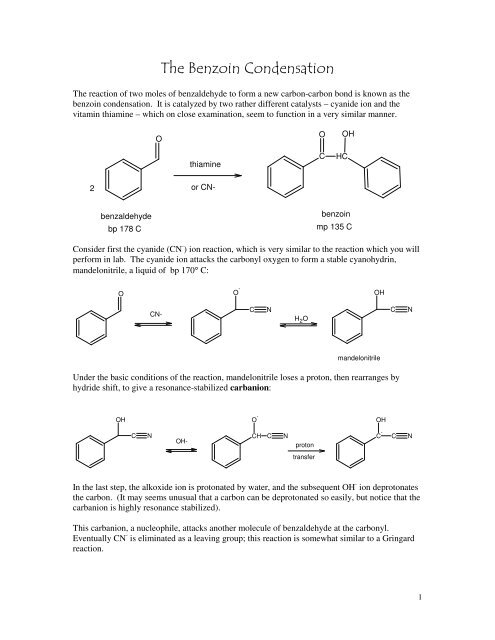
<strong>The</strong> reaction of two moles of benzaldehyde to form a new carbon-carbon bond is known as the<br />
benzoin condensation. It is catalyzed by two rather different catalysts – cyanide ion and the<br />
vitamin thiamine – which on close examination, seem to function in a very similar manner.<br />
2<br />
benzaldehyde<br />
bp 178 C<br />
O<br />
thiamine<br />
or CN-<br />
O<br />
C<br />
HC<br />
OH<br />
benzoin<br />
mp 135 C<br />
Consider first the cyanide (CN - ) ion reaction, which is very similar to the reaction which you will<br />
perform in lab. <strong>The</strong> cyanide ion attacks the carbonyl oxygen to form a stable cyanohydrin,<br />
mandelonitrile, a liquid of bp 170° C:<br />
O<br />
CN-<br />
O -<br />
C N<br />
H 2 O<br />
OH<br />
mandelonitrile<br />
Under the basic conditions of the reaction, mandelonitrile loses a proton, then rearranges by<br />
hydride shift, to give a resonance-stabilized carbanion:<br />
OH<br />
C N<br />
OH-<br />
O -<br />
CH<br />
C N C -<br />
proton<br />
transfer<br />
OH<br />
C N<br />
C N<br />
In the last step, the alkoxide ion is protonated by water, and the subsequent OH - ion deprotonates<br />
the carbon. (It may seems unusual that a carbon can be deprotonated so easily, but notice that the<br />
carbanion is highly resonance stabilized).<br />
This carbanion, a nucleophile, attacks another molecule of benzaldehyde at the carbonyl.<br />
Eventually CN - is eliminated as a leaving group; this reaction is somewhat similar to a Gringard<br />
reaction.<br />
1
Although the benzoin condensation proceeds rapidly and produces benzoin in high yield when<br />
catalyzed by cyanide, we will not perform the reaction with this catalyst. Cyanide is extremely<br />
toxic and accidental exposure to even small amounts can have serious consequences. Cyanide is<br />
not readily absorbed through the skin, but any small cut or break in the skin can lead to accidental<br />
exposure. In solid form, it may also be inhaled in the form of dust or small particles.<br />
A number of biochemical reactions bear a close resemblance to the benzoin condensation but are<br />
not catalyzed by the highly toxic cyanide ion. In the 1960s it was found that that vitamin B1,<br />
thiamine hydrochloride, in the form of the coenzyme thiamine pyrophosphate, can function in a<br />
manner completely analogous to cyanide ion in promoting reactions like the benzoin<br />
condensation. <strong>The</strong> resonance stabilized conjugate base of the thiazolium ion, thiamine, and the<br />
resonance stabilized carbanion that it forms are the key to the reaction. Like the cyanide ion, the<br />
thiazolium ion has just the right balance of nucleophilicity, ability to stabilize the intermediate<br />
anion, and good leaving group qualities.<br />
H3C N NH2<br />
N<br />
H<br />
S<br />
CH 2<br />
N +<br />
Cl -<br />
CH 3<br />
OH<br />
HO -<br />
H3C N NH2<br />
N<br />
C -<br />
S<br />
CH 2<br />
N +<br />
Cl -<br />
thiamine hydrochloride thiamine (a nucleophile)<br />
Note that one of the carbons in the 5-membered ring is a carbanion; this will attack benzaldehyde<br />
in much the same way cyanide does, resulting in a carbanion at the benzyl position of<br />
benzaldehyde. It is thiamine we will use as a catalyst for the benzoin condensation. We will<br />
form the thiamine in situ by adding thiamine hydrochloride and NaOH to our reaction mixture.<br />
<strong>The</strong> importance of thiamine is evident in that it is a vitamin, an essential substance that must be<br />
provided in the diet to prevent beriberi, a nervous system disease.<br />
CH 3<br />
OH<br />
2
Procedure<br />
Do NOT work in pairs or groups for this lab – everyone should work independently!<br />
Place 2.6 grams of thiamine hydrochloride* in a 125 mL Erlenmeyer flask, dissolve it in 8 mL of<br />
water, and add 30 mL of 95% ethanol. Cool the mixture in an ice bath. Add 5 mL of 3 M NaOH<br />
dropwise, with stirring. Keep the mixture cool during this process; the temperature should not<br />
exceed 20°C. You will notice the formation of a yellow color as the thiamine hydrochloride is<br />
deprotonated to form the yellow compound thiamine.<br />
* note: thiamine hydrochloride may be labeled as “Vitamin B1 Hydrochloride”<br />
To the yellow solution, add 15 mL pure* benzaldehyde and cover the flask with aluminum foil.<br />
Heat the mixture, in a warm water bath, at approximately 60°C for 45 minutes. (<strong>The</strong><br />
concentration of the thiamine will increase during this heating process, giving the mixture a<br />
darker yellow or orange color). <strong>The</strong> progress of the reaction can be monitored by TLC.<br />
Alternatively, the reaction mixture can be stored at room temperature for at least 24 hours.<br />
* note: Benzaldehyde, when stored for long periods, may slowly oxidize in air to form<br />
benzoic acid. If the benzaldehyde appears yellow, or if crystals of benzoic acid are<br />
evident, the benzaldehyde should be shaken in a separatory funnel with equal volumes of<br />
5% sodium carbonate until the evolution of CO2 (bubbling or fizzing) no longer occurs.<br />
<strong>The</strong> upper layer (benzaldehyde) can then be dried with calcium chloride or magnesium<br />
sulfate and used. This purification process is not necessary if the benzaldehyde is clear<br />
and free of crystals!<br />
*note: your instructor may have this experiment as a two-week lab. If you are going to<br />
let the reaction mixture sit overnight for the reaction to complete, this will be the point at<br />
which you stop!<br />
Cool the mixture in an ice bath. If crystallization does not occur, withdraw a drop of solution on<br />
a stirring rod and rub it against the inside surface of the flask to induce crystallization. Collect<br />
the product by vacuum filtration and wash it free of the yellow mother liquor with distilled or<br />
de-ionized water. Weigh the crude product. Determine the melting point of the crude product.<br />
<strong>The</strong> benzoin should be colorless and of sufficient purity (mp 134-135°C) to use in subsequent<br />
reactions; usual yield is 10 – 12 grams.<br />
Cleaning Up<br />
Your instructor will collect the synthesized benzoin for use in next week’s experiment. Place the<br />
benzoin in an open beaker, labeled with the contents and your name. You may wish to weigh the<br />
labeled beaker first. Wait until the second week to weigh your sample and measure the<br />
melting point; this will give a chance for the crystals to dry completely.<br />
All reaction by-products and filtrates can be washed down the sink, diluting with water.<br />
3
Post-Lab Questions<br />
1. A procedure is provided to remove benzoic acid, if present, from the benzaldehyde.<br />
a. Why might the presence of benzoic acid be deleterious to the reaction? Fully explain<br />
your answer – be specific!<br />
b. How does treatment with sodium carbonate remove the benzoic acid? Show the<br />
balanced reaction which would occur in that step.<br />
2. Calculate the theoretical yield and % yield of your crude benzoin product. To receive credit,<br />
you must show your work for both calculations; the density of benzaldehyde is 1.045 g/mL.<br />
3. How many π-electrons are in the thiazoline ring (the 5-membered ring) of thiamine<br />
hydrochloride? of thiamine? Are these rings anti-aromatic, non-aromatic, or aromatic?<br />
4. Show a detailed mechanism for the reaction of the thiamine carbanion with benzaldehyde.<br />
<strong>The</strong> reaction will be similar to nucleophilic attacks at carbonyls that you have previously studied<br />
(ie., a Grignard reaction); you may wish to refer to your textbook. (hint: when thiamine reacts<br />
with benzaldehyde, it forms a carbanion very similar in structure to the carbanion of<br />
mandelonitrile, shown on the first page of this handout).<br />
4
Data / Observations Page <strong>Benzoin</strong> <strong>Condensation</strong><br />
Name ____________________________________________________________<br />
Starting materials:<br />
Mass of Erlenmeyer Flask: _____________________ grams<br />
Mass of Erlenmeyer Flask + thiamine HCl: _____________________ grams<br />
Mass of thiamine HCl: _____________________ grams<br />
Product:<br />
Mass of container: _____________________ grams<br />
Mass of container + <strong>Benzoin</strong> _____________________ grams<br />
Mass of <strong>Benzoin</strong> (product): _____________________ grams<br />
melting point of product: _____________________ °C<br />
5
Pre-Lab Questions <strong>Benzoin</strong> <strong>Condensation</strong><br />
Name __________________________________________________<br />
1. What purpose does the NaOH serve in the thiamine catalyzed benzoin condensation?<br />
2. A carbanion is formed when mandelonitrile reacts with NaOH and then undergoes a proton<br />
transfer; the resulting carbanion is described in the discussion as “resonance stabilized”. Draw<br />
the resonance forms of the carbanion; there are several of them. (hint: the negative charge can be<br />
shared with nitrogen, and with the aromatic ring).<br />
3. Calculate the theoretical yield of benzoin, in grams, assuming you begin with 15.0 mL of<br />
benzaldehyde. <strong>The</strong> density of benzaldehyde is 1.045 g/mL. (You should record this value before<br />
you hand in the pre-lab!). You must show your work to receive credit on this problem.<br />
6