1 Chemistry 4420 Dr. Y. Zhao Topic 8 Pericyclic Reactions
1 Chemistry 4420 Dr. Y. Zhao Topic 8 Pericyclic Reactions
1 Chemistry 4420 Dr. Y. Zhao Topic 8 Pericyclic Reactions
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
<strong>Chemistry</strong> <strong>4420</strong> <strong>Dr</strong>. Y. <strong>Zhao</strong><br />
<strong>Topic</strong> 8 <strong>Pericyclic</strong> <strong>Reactions</strong><br />
• Classes of Pericylcic <strong>Reactions</strong><br />
• Electrocyclic <strong>Reactions</strong><br />
• Cylcoaddition and Cycloreversion <strong>Reactions</strong><br />
• Sigmatropic Rearrangements<br />
• Ene <strong>Reactions</strong><br />
1. Classes of <strong>Pericyclic</strong> <strong>Reactions</strong><br />
<strong>Pericyclic</strong> reactions refer to the reactions in which bonds<br />
are formed and broken at the termini of one or more<br />
conjugated π-systems. The electrons move around in a<br />
circle, all bonds are made and broken simultaneously.<br />
There is no intermediates intervene (concertedness).<br />
<strong>Pericyclic</strong> reactions can be classified in four types:<br />
(a) Electrocyclic reactions (ring opening or closing)<br />
(b) Cylcoaddition and Cylcoreversion reactions<br />
(c) Sigmatropic Rearrangement<br />
(d) Ene <strong>Reactions</strong><br />
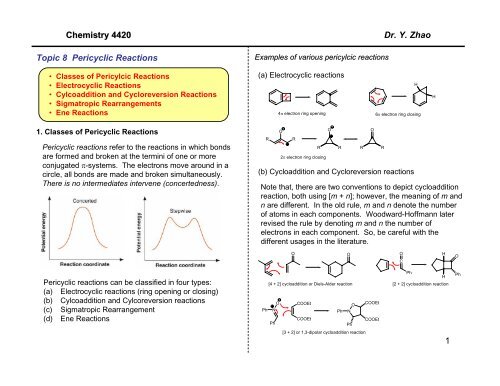
Examples of various pericylcic reactions<br />
(a) Electrocyclic reactions<br />
R<br />
4π electron ring opening 6π electron ring closing<br />
O<br />
R<br />
2π electron ring closing<br />
O<br />
R R<br />
O<br />
R R<br />
(b) Cycloaddition and Cycloreversion reactions<br />
Note that, there are two conventions to depict cycloaddition<br />
reaction, both using [m + n]; however, the meaning of m and<br />
n are different. In the old rule, m and n denote the number<br />
of atoms in each components. Woodward-Hoffmann later<br />
revised the rule by denoting m and n the number of<br />
electrons in each component. So, be careful with the<br />
different usages in the literature.<br />
O O<br />
Ph<br />
H<br />
[4 + 2] cycloaddition or Diels-Alder reaction [2 + 2] cycloaddition reaction<br />
O COOEt<br />
O<br />
COOEt<br />
Ph N<br />
Ph N<br />
Ph<br />
COOEt<br />
Ph<br />
COOEt<br />
[3 + 2] or 1,3-dipolar cycloaddition reaction<br />
O<br />
C<br />
H<br />
H<br />
H<br />
1<br />
O<br />
Ph
<strong>Chemistry</strong> <strong>4420</strong> <strong>Dr</strong>. Y. <strong>Zhao</strong><br />
Other cycloadditions including [8 +2], [4 + 3] and [6 + 4]<br />
cycloadditions are also known. A special class<br />
cycloadditions where one of the component is a single<br />
atom are called cheletropic reactions. For example, [2 +1]<br />
and retro-[4 + 1] cycloadditions.<br />
H<br />
Cl<br />
Cl<br />
C<br />
Cl Cl<br />
H<br />
[2 + 1] cycloaddition or carbene insertion<br />
Ph<br />
Ph<br />
O<br />
Ph<br />
Ph<br />
Ph<br />
Retr o-[4 + 1] cycload dition<br />
Ph<br />
Ph Ph<br />
(c) Sigmatropic Rearrangements<br />
Sigmatropic rearrangements involve the cleavage of a σbond<br />
connecting the end of one fragment with the end of<br />
another, with concerted formation of another σ-bond at the<br />
other ends of the fragments. Like cycloaddition,<br />
sigamtropic rearrangement also uses [m + n] to indicate<br />
the atoms involved in each component.<br />
O<br />
Ph<br />
O<br />
[ 3,3] sigmatropic rearrangement<br />
or Oxy-Cope rearrangement<br />
R O<br />
R O<br />
[3,3] sigmatropic rearrangement<br />
or Claisen rearrangement<br />
OBn<br />
H<br />
H<br />
H<br />
H<br />
[1,5] sigmatropic rearrangement<br />
Ph<br />
H<br />
OBn<br />
Why [3,3]?<br />
O<br />
1 2<br />
bond to be broken 1' 3' Ph bond to be formed<br />
2'<br />
R<br />
HO<br />
H<br />
3<br />
Cl<br />
Cl<br />
H<br />
[1,2] sigmatropic rearrangement<br />
or 1,2-alkyl shift<br />
H<br />
H<br />
H<br />
CH3<br />
H3C<br />
[1,3] sigmatropic rearrangement<br />
(d) Ene <strong>Reactions</strong><br />
H<br />
H<br />
H<br />
O<br />
S<br />
Ph<br />
O<br />
S<br />
Ph<br />
[2,3] sigmatropic rearrangement<br />
The ene reaction is always a 6-electron reaction. It shares<br />
some characteristics with the [4 + 2] cycloaddition and the<br />
[1,5] sigmatropic rearrangement.<br />
H<br />
H<br />
Alder ene reaction<br />
H<br />
O<br />
Se Ph<br />
O<br />
H<br />
H<br />
retro-hetero-ene reaction<br />
O +<br />
Ph<br />
H O<br />
O<br />
Se Ph<br />
Summary of <strong>Pericyclic</strong> <strong>Reactions</strong><br />
electrocyclic reaction<br />
one π bond one σ bond<br />
sigmatropic rearrangement<br />
oneσ bond new σ bond<br />
H<br />
O<br />
Ph<br />
retro-ene reaction<br />
electrocyclic reaction<br />
two π bond two σ bond<br />
H H<br />
ene reaction<br />
oneπ bond one σ bond<br />
andoneσ bond migrate<br />
+<br />
H<br />
O<br />
O<br />
2
<strong>Chemistry</strong> <strong>4420</strong> <strong>Dr</strong>. Y. <strong>Zhao</strong><br />
• Some Features of <strong>Pericyclic</strong> <strong>Reactions</strong><br />
(a) <strong>Pericyclic</strong> reactions are stereospecific<br />
CH3 CH3 175<br />
H<br />
H<br />
oC CH 3<br />
H<br />
H<br />
CH3 CH3 H 175<br />
H<br />
CH3 oC Stereospecificity (stereospecific): A reaction is termed<br />
stereospecific if starting materials differing only in their<br />
configuration are converted into stereoisomeric products.<br />
According to this definition, a stereospecific process is<br />
necessarily stereoselective but not all stereoselective<br />
processes are stereospecific. Stereospecificity may be total<br />
(100%) or partial. The term is also applied to situations where<br />
reaction can be performed with only one stereoisomer. For<br />
example, the exclusive formation of trans-1,2-dibromocyclohexane<br />
upon bromination of cyclohexene is a<br />
stereospecific process, although the analogous reaction with<br />
(E)-cyclohexene has not been performed.<br />
(b) <strong>Pericyclic</strong> reactions are dependent on conditions<br />
CH 3<br />
CH3 H<br />
H<br />
CH 3<br />
H<br />
H<br />
CH 3<br />
2. Electrocylcic <strong>Reactions</strong><br />
hv<br />
CH 3<br />
H<br />
H<br />
CH 3<br />
• Conrotatory and Distrotatory Processes<br />
H 3C CH 3<br />
H<br />
H<br />
CH 3 H<br />
conrotatory (rotate in the same direction)<br />
H<br />
CH 3<br />
H 3C CH 3<br />
H<br />
H<br />
H 3C<br />
CH 3<br />
CH 3<br />
H<br />
H<br />
H H<br />
disrotatory (rotate in different directions)<br />
CH 3<br />
The terms were coined by Woodward and Hoffmann in 1965.<br />
The Woodward-Hoffmann Woodward Hoffmann Rules<br />
4n π e<br />
4n + 2 π e<br />
Ground state<br />
(thermal)<br />
Conrotatory<br />
Disrotatory<br />
Excited state<br />
(photochemical)<br />
Disrotatory<br />
Conrotatory<br />
• Explanations for the Woodward-Hoffmann Rules<br />
(a) FMO Theory (Fukui)<br />
(b) Aromatic Transition States (Dewar-Zimmerman)<br />
(c) Conservation of Orbital Symmetry (Woodward-Hoffmann)<br />
FMO Approach<br />
A<br />
B A<br />
B<br />
disrotatory<br />
Ψ HOMO σ<br />
Lobes with the same phase attract attract each each other other to form form a<br />
new bond; lobes with opposite phases repel repel each other.<br />
A<br />
B A<br />
B<br />
conrotatory<br />
Ψ HOMO σ<br />
A<br />
B<br />
A<br />
B<br />
B<br />
A<br />
A<br />
B<br />
4n + 2 π<br />
4n π<br />
3
<strong>Chemistry</strong> <strong>4420</strong> <strong>Dr</strong>. Y. <strong>Zhao</strong><br />
The FMO approach is very useful and simple; however, it is<br />
not satisfactory and exact theoretically as only the FMOs are<br />
considered.<br />
Aromatic Transition States (the Dewar-Zimmerman Dewar Zimmerman model)<br />
Hückel transition state: the p orbitals around a ring have<br />
zero or an even number of phase inversions.<br />
Möbius transition state: the p orbitals around a ring have<br />
an odd number of phase inversions.<br />
In a Hückel system, 4n +2 electrons, aromatic; 4n<br />
electrons, antiaromatic.<br />
In a Möbius system, 4n electrons, aromatic; 4n + 2<br />
electrons, antiaromatic.<br />
Hence, the Woodward-Hoffmann rules can also be<br />
phrased as: <strong>Reactions</strong> are allowed if they proceed by<br />
aromatic transition states and are forbidden if they<br />
proceed by antiaromatic transition states. states<br />
How to apply the Dewar-Zimmerman model?<br />
A B A B<br />
A B A B<br />
A<br />
B<br />
disrotatory<br />
A<br />
B<br />
Zero phase inversion<br />
Hückel topology<br />
4 e, antiaromatic<br />
Forbidden<br />
A B A B<br />
A B A B<br />
A<br />
B<br />
conrotatory<br />
A<br />
B<br />
One phase inversion<br />
Möbius topology<br />
4 e, aromatic<br />
Allowed<br />
(1) <strong>Dr</strong>aw all the p, s and sp 3<br />
hybridized orbitals (orbital<br />
phases can be assigned<br />
arbitrarily).<br />
(2) Connect all the orbitals that<br />
interact in the starting materials<br />
before the reaction begins.<br />
(3) Allow the reaction proceed<br />
to a postulated transition state.<br />
(4) Connect the lobes that<br />
begin to interact.<br />
(5) Determine the aromatic or<br />
antiaromatic transition state.<br />
Note: the Dewar-Zimmerman is probably the easiest model used<br />
to explain pericyclic reactions. For further reading, see. H. E.<br />
Zimmerman, Acc. Chem. Res. 1971, 4, 272.<br />
Conservation of Orbital Symmetry–Correlation Symmetry Correlation Diagrams<br />
The correlation diagram approach was first propsed by Longuet-<br />
Higgins and Abrahamson a few years after the original paer by<br />
Woodward and Hoffmann. [JACS, 1965, 87, 2045]<br />
The principle of conservation of orbital symmetry says each<br />
orbital of the starting material must be converted to an orbital<br />
with the same symmetry.<br />
4
<strong>Chemistry</strong> <strong>4420</strong> <strong>Dr</strong>. Y. <strong>Zhao</strong><br />
Take the ring closing of butadiene under thermal conditions<br />
as an example,<br />
[2 + 2]<br />
C2 Transition state for a conrotatory<br />
reaction. Symmetry around the<br />
mirror plan is not maintained, but<br />
symmetry around the axis of<br />
rotation is maintained.<br />
Orbital symmetry<br />
σ<br />
Transition state for a disrotatory<br />
reaction. Symmetry around the<br />
mirror plan is maintained, but<br />
symmetry around the axis of<br />
rotation is not maintained.<br />
σ 2 *<br />
π 2 *<br />
π 1<br />
σ 1<br />
Conrotatory interconversion<br />
Thermally allowed<br />
5<br />
π 4 *<br />
π 3 *<br />
π 2<br />
π 1
<strong>Chemistry</strong> <strong>4420</strong> <strong>Dr</strong>. Y. <strong>Zhao</strong><br />
Disrotatory interconversion<br />
Thermally forbidden<br />
Correlation diagram for the conrotatory pathway<br />
Correlation diagram for the disrotatory pathway<br />
6