Partial Periodic Table - Organic Chemistry at CU Boulder ...
Partial Periodic Table - Organic Chemistry at CU Boulder ...
Partial Periodic Table - Organic Chemistry at CU Boulder ...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
CHEMISTRY 3311, Fall 2004<br />
Professor Walba<br />
First Hour Exam, September 23<br />
scores:<br />
1) 26<br />
2) 20<br />
3) 20<br />
4) 18<br />
5) 16<br />
100<br />
<strong>CU</strong> Honor Code Pledge: On my honor, as a University of Colorado <strong>at</strong><br />
<strong>Boulder</strong> Student, I have neither given nor received unauthorized assistance.<br />
Name (printed): _________________________________<br />
Key<br />
Sign<strong>at</strong>ure: ______________________________________<br />
Recit<strong>at</strong>ion TA Name: _____________________________<br />
Recit<strong>at</strong>ion day and time: ___________________________<br />
This is a closed-book exam. The use of notes, models,<br />
calcul<strong>at</strong>ors, scr<strong>at</strong>ch paper, or any other paraphernalia will not be<br />
allowed during the exam. Please put all your answers on the<br />
test. Use the backs of the pages for scr<strong>at</strong>ch.<br />
PLEASE read the questions carefully!<br />
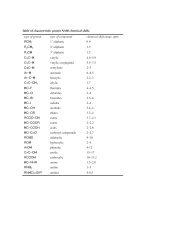
<strong>Partial</strong> <strong>Periodic</strong> <strong>Table</strong><br />
1A<br />
8A<br />
1<br />
H<br />
2A 3A 4A 5A 6A 7A<br />
2<br />
He<br />
3<br />
Li<br />
4<br />
Be<br />
5<br />
B<br />
6<br />
C<br />
7<br />
N<br />
8<br />
O<br />
9<br />
F<br />
10<br />
Ne<br />
11 12 13 14 15 16 17 18<br />
Na Mg Al Si P S Cl Ar<br />
35<br />
Br<br />
53<br />
I
Name:<br />
1) (26 pts) a) Draw a valid valence bond structure for each of the possible isomers (both constitutional<br />
isomers and stereoisomers) with the molecular formula C 3H 5F. Draw each isomer ONLY ONCE.<br />
b) Draw a valid valence bond structure for each of the following molecules. Carefully show all lone pairs,<br />
unpaired electrons, and formal charges in your structures.<br />
H<br />
CH 4 CH 3 (CH 3) + (CH 3) -<br />
H<br />
C<br />
F<br />
H<br />
H<br />
F<br />
c) For 1,2-butadiene, indic<strong>at</strong>e the hybridiz<strong>at</strong>ion of each carbon. Put your answer for each carbon in the box to<br />
the right its number.<br />
H<br />
C C C<br />
H 1 2 3<br />
H<br />
H<br />
CH 3<br />
4<br />
C<br />
H<br />
H<br />
F<br />
1<br />
2<br />
F<br />
H<br />
Page 2 of 7<br />
C<br />
H<br />
H<br />
H<br />
F<br />
C<br />
sp 3<br />
2 sp2 sp 4 sp3 H<br />
H
Name:<br />
2) (20 pts) a) Circle the stronger Brønsted acid in each of the following pairs of structures.<br />
H 2O<br />
CH 3NH 2<br />
H<br />
H<br />
H 3O +<br />
CH 3CH 3<br />
H<br />
H<br />
b) Circle the compound with the more stable carbonyl group in each of the following pairs of compounds.<br />
O<br />
H 3C H<br />
O<br />
H 3C CH 3<br />
O O<br />
O<br />
O<br />
O<br />
Page 3 of 7<br />
NH 3<br />
OH<br />
OH<br />
NH 2<br />
OH<br />
O<br />
O O<br />
O<br />
OH 2<br />
N<br />
H
Name:<br />
3) (20 pts) a) Give the stereochemical descriptor (E or Z) for the following alkenes.<br />
b) Circle the arom<strong>at</strong>ic compounds in the following list.<br />
B<br />
H<br />
H<br />
F<br />
N<br />
H<br />
Z E<br />
Page 4 of 7<br />
H<br />
F OH<br />
N<br />
H H H H<br />
c) I have given a valence bond structure of N-methylacetamide below. Give the two additional major<br />
resonance contributors to the structure. Be sure to show all lone pairs and formal charges in your structures.<br />
O<br />
H 3C N CH 3<br />
H<br />
O<br />
H 3C N CH 3<br />
H<br />
O<br />
H 3C N CH 3<br />
d) N-methylacetamide has a higher boiling point than N,N-dimethylacetamide. In one short sentence, explain<br />
why this is the case.<br />
O<br />
H 3C N CH 3<br />
H<br />
boiling point = 205°C<br />
O<br />
H 3C N CH 3<br />
CH 3<br />
boiling point = 166°C<br />
N-methylacetamide has a higher boiling point because it can particip<strong>at</strong>e in intermolecular hydrogen bonding.<br />
H
Name:<br />
4) (18 pts) a) Circle the compound with the higher he<strong>at</strong> of combustion (th<strong>at</strong> is, the one th<strong>at</strong> gives off the most<br />
he<strong>at</strong> when you burn it in oxygen).<br />
b) On the following energy diagram, very clearly indic<strong>at</strong>e the rel<strong>at</strong>ive energy of the two isomers given in part<br />
4a by drawing a short horizontal line above each structure indic<strong>at</strong>ing its energy. Make sure it’s easy to see your<br />
lines, and th<strong>at</strong> it’s clear which isomer has higher energy.<br />
E<br />
c) For each of the following pairs of alcohols, circle the one th<strong>at</strong> would react faster with Lucas reagent.<br />
OH<br />
OH<br />
Page 5 of 7<br />
OH OH
4) – continued<br />
Name:<br />
d) Propose an arrow-pushing mechanism for the following transform<strong>at</strong>ion. Assume for this purpose th<strong>at</strong> the<br />
reagent [ZnCl 2, HCl, H 2O] = H + . Show only one valence bond structure for each intermedi<strong>at</strong>e in your<br />
mechanism, but be sure to give all the intermedi<strong>at</strong>es.<br />
OH<br />
H +<br />
ZnCl2, HCl, H2O OH Cl<br />
e) [Note – this is a hard question, but hay, it’s only five points. However, it might be a good idea to finish the<br />
rest of the exam before you tackle this question] When the 3-methyl-2-butanol reacts with Lucas reagent, two<br />
isomeric products are formed, as shown below. Using resonance structures, show how this happens.<br />
ZnCl2, HCl, H2O OH Cl<br />
O<br />
Cl or Cl<br />
H<br />
Page 6 of 7<br />
H + H 2O<br />
Cl<br />
+<br />
Cl
Name:<br />
5) (16 pts) Give the single major organic product of each of the following reactions. In both cases, only one<br />
product is formed, in high yield. Assume th<strong>at</strong> there is excess hydrogen gas (as usual in a c<strong>at</strong>alytic<br />
hydrogen<strong>at</strong>ion), but th<strong>at</strong> the reaction is only allowed to proceed for a few hours <strong>at</strong> room temper<strong>at</strong>ure.<br />
a)<br />
b)<br />
H<br />
N O<br />
H<br />
H<br />
H 2, Pt<br />
room temp<br />
H 2, Pt<br />
room temp<br />
Page 7 of 7<br />
H<br />
H<br />
N O<br />
c) Penicillin was the first important antibiotic (it kills bacteria). Unfortun<strong>at</strong>ely, many bacteria have developed<br />
resistance to penicillin by making an enzyme called penicillinase. For our purposes, penicillinase is a giant<br />
molecule with a nucleophile on it, as indic<strong>at</strong>ed below. Penicillin has two amide groups, with the amide<br />
carbonyls labeled 1 and 2 on the structure. The penicillinase nucleophile <strong>at</strong>tacks one of those carbonyls, which<br />
ends up making the penicillin harmless to the bacteria. A key point is th<strong>at</strong> one of the carbonyl groups is much<br />
more reactive than the other.<br />
1<br />
O<br />
H<br />
N<br />
O<br />
N<br />
2<br />
Penicillin<br />
S<br />
CO 2H<br />
Penicillinase—Nu<br />
c) Which carbonyl group is the most reactive one? #2<br />
d) Propose a structure for the intermedi<strong>at</strong>e formed when the penicillinase—Nu: - reacts with penicillin.<br />
H<br />
O<br />
N<br />
Penicillinase—Nu<br />
O<br />
N<br />
S<br />
CO 2H<br />
H