Experiment 3 - Organic Chemistry at CU Boulder
Experiment 3 - Organic Chemistry at CU Boulder
Experiment 3 - Organic Chemistry at CU Boulder
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
<strong>Experiment</strong> 3<br />
Distill<strong>at</strong>ion: Separ<strong>at</strong>ion of a Mixture of Methyl Acet<strong>at</strong>e and Propyl Acet<strong>at</strong>e<br />
Reading: Handbook for <strong>Organic</strong> <strong>Chemistry</strong> Lab, Sections on Simple Distill<strong>at</strong>ion, Fractional Distill<strong>at</strong>ion, and Gas<br />
Chrom<strong>at</strong>ography.<br />
Distill<strong>at</strong>ion is the process of vaporizing a liquid, condensing the vapor, and collecting the condensed<br />
liquid (or condens<strong>at</strong>e) in a different container. It is a general technique th<strong>at</strong> permits liquid compounds to<br />
be purified or solvents to be selectively removed from non-vol<strong>at</strong>ile m<strong>at</strong>erials. Simple, fractional, steam,<br />
and vacuum distill<strong>at</strong>ion are four modific<strong>at</strong>ions of the basic distill<strong>at</strong>ion technique.<br />
If a perfect separ<strong>at</strong>ion of two components A and B is achieved during a distill<strong>at</strong>ion, a plot of<br />
temper<strong>at</strong>ure vs volume of condens<strong>at</strong>e looks like the ideal graph (Figure 3.1). The entire lower boiling<br />
component A distills <strong>at</strong> its boiling point until it is removed from the mixture; then, the higher boiling<br />
component B distills <strong>at</strong> its boiling point. Ideal separ<strong>at</strong>ions are not achieved when separ<strong>at</strong>ing mixtures of<br />
compounds with boiling points closer together than 10°C. This is because the component B has an<br />
appreciable vapor pressure <strong>at</strong> the boiling point of component A. In a labor<strong>at</strong>ory situ<strong>at</strong>ion, one can plot<br />
the volume of distill<strong>at</strong>e vs. temper<strong>at</strong>ure of the distilling vapor to determine how closely a distill<strong>at</strong>ion<br />
resembles an ideal separ<strong>at</strong>ion.<br />
temper<strong>at</strong>ure ˚C<br />
pure B begins to distil<br />
boiling point of pure B<br />
pure A begins to distil<br />
volume of distill<strong>at</strong>e<br />
“curve” for an ideal distill<strong>at</strong>ion<br />
curve for simple distill<strong>at</strong>ion<br />
curve for fractional distill<strong>at</strong>ion<br />
boiling point of pure A<br />
Figure 3.1 » Ideal, fractional and simple distill<strong>at</strong>ion curves.<br />
In this experiment, you will use the techniques of simple and fractional distill<strong>at</strong>ion to separ<strong>at</strong>e two<br />
liquids, methyl acet<strong>at</strong>e and propyl acet<strong>at</strong>e. The structures of these compounds are shown below (Figure<br />
3.2). Both compounds are esters based on acetic acid; the acidic hydrogen in acetic acid is replaced with<br />
an methyl group or a propyl group respectively.<br />
Figure 3.2 » Structures of methyl acet<strong>at</strong>e and propyl acet<strong>at</strong>e, and their parent compound, acetic acid<br />
13
<strong>Experiment</strong> 3: Distill<strong>at</strong>ion<br />
You will compare the efficiencies of the separ<strong>at</strong>ions achieved in the two distill<strong>at</strong>ion techniques, both by<br />
monitoring the temper<strong>at</strong>ure of the condensing vapors as a function of the quantity distilled and by<br />
analyzing the composition of two samples of the distill<strong>at</strong>e by gas chrom<strong>at</strong>ography.<br />
Gas Chrom<strong>at</strong>ography<br />
Gas chrom<strong>at</strong>ography (GC or GLC) is an important chrom<strong>at</strong>ographic technique used by the organic<br />
chemist. In the gas chrom<strong>at</strong>ograph an inert carrier gas constitutes the moving or mobile phase while a<br />
high-boiling liquid layer deposited on an inert solid support makes up the non-moveable component.<br />
Gas chrom<strong>at</strong>ography is explained in detail in the Handbook for <strong>Organic</strong> <strong>Chemistry</strong>.<br />
Safety Precautions<br />
Methyl acet<strong>at</strong>e and propyl acet<strong>at</strong>e are flammable. Do not distill to dryness, since it can lead to a<br />
potentially hazardous situ<strong>at</strong>ion.<br />
Procedure<br />
In this lab the TA will assign each student either simple or fractional distill<strong>at</strong>ion. During the lab, make arrangements with a student<br />
who is performing the other type of distill<strong>at</strong>ion so you can compare d<strong>at</strong>a in your lab report.<br />
You are to distill 25 mL of a 1:1 (by volume) methyl acet<strong>at</strong>e:propyl acet<strong>at</strong>e mixture using either the<br />
simple or fractional distill<strong>at</strong>ion setup. Your TA will assign you one of the two techniques; however, you<br />
are responsible for coordin<strong>at</strong>ing with another student and making copies of each other’s d<strong>at</strong>a. This way<br />
you can discuss the difference between the two d<strong>at</strong>a sets in your lab write-up. Set up your appar<strong>at</strong>us as<br />
illustr<strong>at</strong>ed in Figure 3.3 (simple distill<strong>at</strong>ion) or Figure 3.4 (fractional distill<strong>at</strong>ion). The fraction<strong>at</strong>ing<br />
column, if you need one, is available from your TA. This is a condenser with glass prongs <strong>at</strong> the inside<br />
bottom, to hold a stack of glass beads in place. Please do not allow these glass beads to fall out of the<br />
column – they can be a tripping hazard if they are on the floor.<br />
Place a he<strong>at</strong>ing mantle under your 100mL round bottom flask. Plug the he<strong>at</strong>ing mantle into a Variac—<br />
never plug a he<strong>at</strong>ing mantle directly into an electrical outlet—but do not turn the Variac on yet. Ensure<br />
th<strong>at</strong> the thermometer bulb is just below the bend of the Y-adaptor.<br />
If you are performing a fractional distill<strong>at</strong>ion, the appar<strong>at</strong>us will be taller and you may have some<br />
difficulty fitting it into your fume hood. It will still fit if you: 1) Rot<strong>at</strong>e the ring stand base to one side, so<br />
the he<strong>at</strong>ing mantle is resting directly on the bench, 2) Before inserting the thermometer, slide it down<br />
through the thermometer adapter, so it’s temporarily shorter, 3) Tilt the entire setup towards you slightly<br />
and insert the thermometer, then rot<strong>at</strong>e the setup back into place. Remember to slide the thermometer<br />
back up, so the bulb is in the right position.<br />
Place 25 mL of the 1:1 methyl acet<strong>at</strong>e:propyl acet<strong>at</strong>e mixture in the round bottom flask; don’t forget to<br />
add boiling chips to this flask.<br />
Set the Variac to 50, and then turn on the Variac power. When the mixture begins boiling, adjust the<br />
Variac setting as necessary so th<strong>at</strong> the distill<strong>at</strong>e collects <strong>at</strong> a r<strong>at</strong>e of 1–2 drops/sec.<br />
As the distill<strong>at</strong>ion proceeds, you should collect the liquid in a 10 mL gradu<strong>at</strong>ed cylinder. Record the<br />
temper<strong>at</strong>ure of the vapors <strong>at</strong> the distill<strong>at</strong>ion head as a function of the volume of condens<strong>at</strong>e (take a<br />
reading about every 1.0 mL). When 3 mL have been collected, remove the gradu<strong>at</strong>ed cylinder and<br />
substitute it with a sample vial. Collect about 20 drops (0.5 mL); cap and save this sample for GC<br />
analysis (if you fail to cap your vial, your sample will evapor<strong>at</strong>e!) Then, put the 10 mL gradu<strong>at</strong>ed cylinder<br />
back under the vacuum adaptor and continue collecting.<br />
14
<strong>Experiment</strong> 3: Distill<strong>at</strong>ion<br />
Continue recording the temper<strong>at</strong>ure of the vapors every 1.0 mL (You may have to turn up the Variac if<br />
the distill<strong>at</strong>ion slows down during the process; try setting it to 60). When 15 mL have been collected,<br />
again remove the gradu<strong>at</strong>ed cylinder, substitute it with a sample vial, and collect 20 drops. You will have<br />
to empty the 10 mL gradu<strong>at</strong>ed cylinder once.<br />
Discontinue the distill<strong>at</strong>ion after 20 mL have been collected or before the distill<strong>at</strong>ion pot is dry.<br />
round bottom<br />
flask<br />
he<strong>at</strong> source<br />
goes here<br />
thermometer<br />
Y-adaptor<br />
Figure 3.3 » Setup for a simple distill<strong>at</strong>ion. Neither<br />
the clamps nor the Keck clips are shown.<br />
Gas Chrom<strong>at</strong>ography<br />
Figure 3.4 » Setup for a fractional distill<strong>at</strong>ion. Neither<br />
the clamps nor the Keck clips are shown.<br />
Run a Gas Chrom<strong>at</strong>ography trace for each of the samples you collected. The general instructions for<br />
GC will be covered by your TA and are also posted above each GC instrument. Use the following<br />
settings for each run:<br />
Start temper<strong>at</strong>ure 65C<br />
Hold time 1 min<br />
Ramp r<strong>at</strong>e 10C/min<br />
Final temper<strong>at</strong>ure 65C<br />
Hold time 2 min<br />
Total length 6.0 min<br />
Pressure 7.0 kPa<br />
Analysis of D<strong>at</strong>a<br />
w<strong>at</strong>er out<br />
condenser<br />
w<strong>at</strong>er in<br />
receiving flask<br />
goes here<br />
thermometer<br />
adaptor<br />
condensor filled<br />
with glass beads<br />
(available from<br />
your TA)<br />
condenser<br />
vacuum adaptor<br />
1) Input your d<strong>at</strong>a into a spreadsheet program like Excel and use it to gener<strong>at</strong>e a graph of<br />
temper<strong>at</strong>ure vs. volume of distill<strong>at</strong>e for the distill<strong>at</strong>ion you performed. Place the simple and<br />
fractional d<strong>at</strong>a on the same graph. Add to the graph the ideal curve expected for a mixture of<br />
methyl acet<strong>at</strong>e and propyl acet<strong>at</strong>e. If you need help, you can search for “how to cre<strong>at</strong>e a sc<strong>at</strong>ter<br />
plot in Excel” – there are numerous tutorials available online.<br />
2) Decide which peak belongs to which compound in your GC printouts and report the rel<strong>at</strong>ive<br />
w<strong>at</strong>er out<br />
he<strong>at</strong> source<br />
goes here<br />
w<strong>at</strong>er in<br />
receiving flask<br />
goes here<br />
15
<strong>Experiment</strong> 3: Distill<strong>at</strong>ion<br />
16<br />
compositions of the mixtures.<br />
Wastes<br />
Place the pot residue, all distill<strong>at</strong>ion fractions, and the GC samples in the “Recovered Distill<strong>at</strong>e” bottle<br />
in the main hood. This mixture of methyl acet<strong>at</strong>e and propyl acet<strong>at</strong>e will be recycled for use in future<br />
labor<strong>at</strong>ory sessions. Make sure th<strong>at</strong> you do not contamin<strong>at</strong>e the recovery bottle with w<strong>at</strong>er or acetone.<br />
Used boiling chips can be placed in the white solid waste bins around the lab.<br />
Study Questions<br />
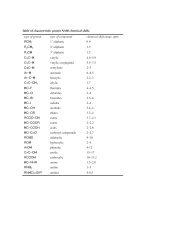
1) In <strong>Boulder</strong>, CO, the <strong>at</strong>mospheric pressure is always less than standard <strong>at</strong>mospheric pressure, and<br />
therefore the observed boiling points will are lower than those reported in the liter<strong>at</strong>ure. The<br />
barometric pressure in <strong>Boulder</strong> is usually around 625mm Hg (625 Torr). Wh<strong>at</strong> will be the<br />
observed boiling points of methyl acet<strong>at</strong>e and propyl acet<strong>at</strong>e? (Refer to the first three pages of<br />
the Distill<strong>at</strong>ion chapter in the Handbook for <strong>Organic</strong> <strong>Chemistry</strong>.)<br />
2) A gas chrom<strong>at</strong>ograph is being used to study a mixture of chlorobutanes. The column is 1.5<br />
meters long and contains 5% silicon elastomer on Chromosorb W; the column temper<strong>at</strong>ure is<br />
60°C, the injector and detector temper<strong>at</strong>ures are 200°C; the carrier gas flow r<strong>at</strong>e is 40 mL/min.<br />
Under these conditions, the retention times of five chlorobutane isomers are:<br />
Compound Retention time<br />
1-chlorobutane 0.4 min<br />
1,1-dichlorobutane 0.95 min<br />
1,2-dichlorobutane 1.1 min<br />
1,3-dichlorobutane 1.3 min<br />
1,4-dichlorobutane 2.3 min<br />
a. A small sample of an unknown is injected into the GC under the identical<br />
conditions listed above, and the GC trace below is obtained (see next page). Wh<strong>at</strong><br />
compounds might be present in the unknown and wh<strong>at</strong> percent of each is present?<br />
START<br />
.61<br />
RT AREA TYPE AR/HT AREA%<br />
0.61 XXXX XX XX 0.009<br />
1.11 XXXX XX XX 55.874<br />
2.27 XXXX XX XX 44.117<br />
TOTAL AREA=XX<br />
MUL FACTOR=XX<br />
2.27<br />
1.11
<strong>Experiment</strong> 3: Distill<strong>at</strong>ion<br />
b. Wh<strong>at</strong> effect would raising the column temper<strong>at</strong>ure of the GC to 90°C have on the<br />
retention times of the compounds in (a)? (Assume all other conditions are identical.)<br />
3) Why does a rapid distill<strong>at</strong>ion th<strong>at</strong> floods the fraction<strong>at</strong>ing column lead to poor separ<strong>at</strong>ion of<br />
components?<br />
4) Two alcohols have a low retention time and are not separable on a silicone GC column. Explain<br />
why they have a longer retention time and are separable on a Carbowax column. (Hint: the<br />
structures and rel<strong>at</strong>ive polarities of the two types of columns are given in the gas<br />
chrom<strong>at</strong>ography section in the Handbook for <strong>Organic</strong> <strong>Chemistry</strong>.)<br />
5) Wh<strong>at</strong> are the pros and cons of rinsing a microsyringe with the mixture to be analyzed, r<strong>at</strong>her<br />
than with a solvent such as acetone or ethanol?<br />
17
<strong>Experiment</strong> 3: Distill<strong>at</strong>ion<br />
18