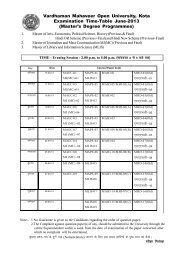
- Page 1 and 2:
(ii) lks'ky usVofdZax lkbV~l (iii)
- Page 3 and 4:
Le`fr dk 'kSf{kd egÙo D;k gS \ 7 6
- Page 5 and 6:
fuEufyf[kr esa ls fdUgha nks ij laf
- Page 7 and 8:
fuEufyf[kr esa ls fdUgha nks ij laf
- Page 9 and 10:
A MAHD-03 ,e- ,- ¼iwokZ)Z½ ijh{kk
- Page 11 and 12:
A B.Ed.-02 B.Ed. EXAMINATION, June,
- Page 13 and 14:
iz.kkyh mikxe dh fo'ks"krkvksa ij f
- Page 15 and 16:
iz'ukoyh i)fr dks ifjHkkf"kr dhft;s
- Page 17 and 18:
8. Why conservation of forest is ne
- Page 19 and 20:
10. Explain why : (i) The melting p
- Page 21 and 22:
A PCO B.C.P. EXAMINATION, June, 201
- Page 23 and 24:
A ISS B.A.P. EXAMINATION, June, 201
- Page 25 and 26:
4. Give a summary of Hazlitt's essa
- Page 27 and 28:
2. Describe in detail the main prin
- Page 29 and 30: 5. vfHkizsj.k dks ifjHkkf"kr djrs g
- Page 31 and 32: companies accounts as per Section 2
- Page 33 and 34: 3. Explain about the mechanism of L
- Page 35 and 36: 2. Explain any two techniques of th
- Page 37 and 38: Plant and machineries 3,00,000 Land
- Page 39 and 40: fofu;ksx 2,00,000 QuhZpj ,oa fQDplZ
- Page 41 and 42: ys[k vkSj Qhpj esa vUrj Li"V dhft;s
- Page 43 and 44: vk/kqfud iqLrdky; O;oLFkk esa ^ikBd
- Page 45 and 46: mnkjhdj.k ds nkSj esa eqfnzr ek/;e
- Page 47 and 48: ckaXykns'k esa izsl dh Lora=rk vkaf
- Page 49 and 50: 2. (i) Calculate Economic Order Qua
- Page 51 and 52: A BC-02 B.Com. (Part I) EXAMINATION
- Page 53 and 54: August 89 September 92 October 102
- Page 55 and 56: mijksä leadksa ls (i) Hkkfjr lewgh
- Page 57 and 58: xq#Roh; {ks= o xq#Roh; foHko dks le
- Page 59 and 60: 5. Comment on the uniqueness of Hop
- Page 61 and 62: l dFku dh foospuk dhft;s rFkk mu if
- Page 63 and 64: lhekUr mi;ksfxrk vkSj dqy mi;ksfxrk
- Page 65 and 66: vd'ks#dh tUrqvksa ds oxhZdj.k dk o.
- Page 67 and 68: ^^vkUrfjd fujh{k.k** ls vki D;k le>
- Page 69 and 70: 1. Discuss various steps of modern
- Page 71 and 72: iqfu lklqj ge xouc dkyhA fdr ge] fd
- Page 73 and 74: iz'ukoyh o vuqlwph esa vUrj crkb;sA
- Page 75 and 76: 20-30 23 30-40 22 40-50 25 50-60 10
- Page 77 and 78: 9. Define classified and dictionary
- Page 79: fuEufyf[kr ij laf{kIr fVIif.k;k¡ f
- Page 83 and 84: gj"kr vuUn eu efg gqykl] yS tq egy
- Page 85 and 86: fuEu L.P.P. dks xzkQ fof/k ls gy dh
- Page 87 and 88: 11. Evaluate : eku Kkr dhft;s % 1 2
- Page 89 and 90: fuEufyf[kr f}?kkr lehdj.k dks gy dh
- Page 91 and 92: 4. Does learning, knowledge and exp
- Page 93 and 94: 4. In what ways is 'Emma' a realist
- Page 95 and 96: A MS-41 M.B.A. EXAMINATION, June, 2
- Page 97 and 98: A BC-06 B.Com. (First Year) EXAMINA
- Page 99 and 100: 10. Write short notes on the follow
- Page 101 and 102: A BC-15 B.Com. (Third Year) EXAMINA
- Page 103 and 104: 10. What is report writing ? Explai
- Page 105 and 106: ^míhiu ifjorZu dkS'ky* ls vki D;k
- Page 107 and 108: 8. Write a note on Lenin's New Econ
- Page 109 and 110: A QEG B.A./B.Com./B.Sc./B.C.A. EXAM
- Page 111 and 112: call________he left for his office.
- Page 113 and 114: (ii) Mr. Verma has decided_____a ca
- Page 115 and 116: ns[krs gSa] ogha iM+s jgrs gSaA che
- Page 117 and 118: vFkok nq%[k dh fiNyh jtuh chp fodlr
- Page 119 and 120: vkidks mi;ksxh cukrh gS] tks vkidks
- Page 121 and 122: 3. eSyk vk¡py ds vk/kkj ij js.kq d
- Page 123 and 124: A MAHD-02 M.A. (Previous) EXAMINATI
- Page 125 and 126: NksVs&NksVs eksrh tSls mlds 'khry r
- Page 127 and 128: tanb ig ds okLrfod ,oa dkYifud Hkk
- Page 129 and 130: 6. Solve the following : (i) z0 1 1
- Page 131 and 132:
nf{k.k&iwoZ ,f'k;kbZ ns'kksa dh vkf
- Page 133 and 134:
vFkok ,p-vkbZ-oh-@,M~l ds bfrgkl ij
- Page 135 and 136:
8. Explain the constitutional devel
- Page 137 and 138:
4. e# fodkl dk;ZØe jktLFkku ds fdu
- Page 139 and 140:
8. izkphu jktLFkkuh x| fo/kkoka lwa
- Page 141 and 142:
fuEufyf[kr ij y?kq fVIif.k;k¡ fyf[
- Page 143 and 144:
(b) How will you obtain starting wi
- Page 145 and 146:
la;qfXer Mkbuksa esa cgqyhdj.k ij f
- Page 147 and 148:
5. What do you mean by product life
- Page 149 and 150:
3. Write various challenges to inte
- Page 151 and 152:
3. "It is never safe to take publis
- Page 153 and 154:
A MAPA-05 M.A. (Final) EXAMINATION,
- Page 155 and 156:
fuEufyf[kr leadksa ls izeki fopyu ,
- Page 157 and 158:
A BC-11 B.Com. (Second Year) EXAMIN
- Page 159 and 160:
A PA-02 B.A. (Part I) EXAMINATION,
- Page 161 and 162:
(iii) ysfol ,- dkstj (iv) vkj- ds-
- Page 163 and 164:
500-600 12 600-700 11 700-800 07 80
- Page 165 and 166:
A MAED-06 M.A. EXAMINATION, June, 2
- Page 167 and 168:
vUrjkZT;h; foLFkkfir deZdkj ¼fu;ks
- Page 169 and 170:
A CLAW-02 C.L.A.W. EXAMINATION, Jun
- Page 171 and 172:
A EEC-04 B.A./B.Com. EXAMINATION, J
- Page 173 and 174:
A CTB- 02 C.T.B. EXAMINATION, June,
- Page 175 and 176:
D;k gksrh gS] le>kb;sA Cell address
- Page 177 and 178:
1. Write down the main features of
- Page 179 and 180:
A EEC-03 B.A. EXAMINATION, June, 20
- Page 181 and 182:
IQ 1 1 2 3 4 5 Before training 110
- Page 183 and 184:
fuEu lehdj.k dks gy dhft;s % 2 x 4
- Page 185 and 186:
Or What is food energy ? How energy
- Page 187 and 188:
,f'k;k esa csgrj thou Lrj izkIr dju
- Page 189 and 190:
Or Describe the main features of th
- Page 191 and 192:
1. Describe the importance of Haldi
- Page 193 and 194:
4. (a) What are physical phenomenon
- Page 195 and 196:
(b) What is the difference between
- Page 197 and 198:
2. Differentiate between : (i) Abso
- Page 199 and 200:
3. (a) Write short notes on : (any
- Page 201 and 202:
(b) Give a brief account of the fin
- Page 203 and 204:
Or Describe the energy scenario in
- Page 205 and 206:
gsrq ty xzg.k ctV dSls tkjh gksrk g
- Page 207 and 208:
2. thou esa uSfrd ewY; O;fä ds O;f
- Page 209 and 210:
4. ifjokj] tkfr ,oa lewg LokLF; dks
- Page 211 and 212:
jktLFkku ds xkSjo fpÙkkSM+ nqxZ ij
- Page 213 and 214:
ysuk pkgrh Fkha] mlesa tks Fkk og H
- Page 215 and 216:
lkekU; ,dy dks ifjHkkf"kr dhft;sA f
- Page 217 and 218:
fo|ky; ikB~;Øe dksBkjh f'k{kk vk;k
- Page 219 and 220:
1. Discuss the industrial developme
- Page 221 and 222:
(b) How to install an external mode
- Page 223 and 224:
iqfyl ,oa tsy vfHkj{kk ds rgr efgyk
- Page 225 and 226:
2. State the various benefits provi
- Page 227 and 228:
2. Explain the importance of Distan
- Page 229 and 230:
Or Describe school health services
- Page 231 and 232:
1. Explain the importance of Metal
- Page 233 and 234:
,d vYi fodflr ns'k esa yksd foÙk u
- Page 235 and 236:
1. Define the scope of internationa
- Page 237 and 238:
1. Examine the success of Paris Pea
- Page 239 and 240:
1. Describe the meaning and importa
- Page 241 and 242:
1. What do you mean by Stock Exchan
- Page 243 and 244:
(iv) Acetone (v) Acetaldehyde fdl i
- Page 245 and 246:
(iv) ,sekbM (v) dhVksu 3. (a) Give
- Page 247 and 248:
fuEufyf[kr vfHkfØ;kvksa dh fØ;kfo
- Page 249 and 250:
(b) What are templates in MS-Word ?
- Page 251 and 252:
3. (a) What is B2B and C2C in E-com
- Page 253 and 254:
Or Discuss the advantages and disad
- Page 255 and 256:
3. Prepare a checklist for identifi
- Page 257 and 258:
4. ikfjHkkf"kd 'kCn ls D;k rkRi;Z g
- Page 259 and 260:
3. [kM+h cksyh ds fofHkUu :iksa dks
- Page 261 and 262:
5. vksfM+;k Hkfä lkfgR; ds izeq[k
- Page 263 and 264:
A EHD-03 B.A. EXAMINATION, June, 20
- Page 265 and 266:
A CFN-03 C.F.N. EXAMINATION, June,
- Page 267 and 268:
10. State the facts and principles
- Page 269 and 270:
3. xHkkZoLFkk ds funku (Diagnosis o
- Page 271 and 272:
1. Explain the recent trends and pr
- Page 273 and 274:
A BC-12 B.Com. (Second Year) EXAMIN
- Page 275 and 276:
special machines. Management does n
- Page 277 and 278:
10. How the pH of a solution is det
- Page 279 and 280:
(iv) vu'o% (v) ?ku';ke% (vi) f'kods
- Page 281 and 282:
¼[k½ ;kSoukjaHks p izk;% 'kkL=tyi
- Page 283 and 284:
(b) What is ergonomics in system de
- Page 285 and 286:
A PGDCA/CCNI/MSCS-05 P.G.D.C.A./M.S
- Page 287 and 288:
(c) Write synonyms of the given wor
- Page 289 and 290:
A PGDESD-05 P.G.D.E.S.D. EXAMINATIO
- Page 291 and 292:
A HD-05 B.A. (Third Year) EXAMINATI
- Page 293 and 294:
mlds O;fäRo dks jkxkfRedrkvksa dks
- Page 295 and 296:
vYi foØsrkf/kdkj esa dher o mRifÙ
- Page 297 and 298:
A CDE-03 C.D.E. EXAMINATION, June,
- Page 299 and 300:
A PGDLL-05 P.G.D.L.L. EXAMINATION,
- Page 301 and 302:
(ii) ukWlMkd (iii) Hkkjr eas vk;qfo
- Page 303 and 304:
fuEufyf[kr esa ls fdUgha nks ij laf
- Page 305 and 306:
Boys 16 36 20 72 Total 34 56 54 144
- Page 307 and 308:
(i) ,fFky ,flVsV (ii) ,flVsfYMgkbM
- Page 309 and 310:
3. LQksV fl)kUr dk ifjp; nsrs gq, /
- Page 311 and 312:
1. Discuss the concept of History a
- Page 313 and 314:
1. Discuss socio-political context
- Page 315 and 316:
vFkok fnu Hkj vdsyh jgdj nsj jkf= r
- Page 317 and 318:
A AFE-06 Certificate in HIV and Fam
- Page 319 and 320:
vFkok ,sfrgkfld :eku ds vk/kkj ij j
- Page 321 and 322:
January 3 Paid to Mohan 2,500 Janua
- Page 323 and 324:
(ii) jktLFkku esa >hysa (iii) jktLF
- Page 325 and 326:
QSDVªh ds ckjs esa vki D;k tkurs g
- Page 327 and 328:
A BT-01 B.Sc. (Part I) EXAMINATION,
- Page 329 and 330:
9. Give two examples of the followi
- Page 331 and 332:
(b) Write a note on Symbiosis. 5 lg
- Page 333 and 334:
fuEufyf[kr esa vUrj Li"V dhft;s % (
- Page 335 and 336:
(b) In her hands she hold the oil l
- Page 337 and 338:
uq rqjax cj otzA otz BsyS otzkluAA
- Page 339 and 340:
10. Write short notes on the follow
- Page 341 and 342:
A CH-05 B.Sc. (Part II) EXAMINATION
- Page 343 and 344:
fuEufyf[kr ij fVIif.k;k¡ fyf[k;s %
- Page 345 and 346:
1. Write a critical note on special
- Page 347 and 348:
1. Describe the literary sources fo
- Page 349 and 350:
fu"kspu D;k gS \ fu"kspu ds fofHkUu
- Page 351 and 352:
1. Discuss the major characteristic
- Page 353 and 354:
1. Discuss the traditional approach
- Page 355 and 356:
1. Determine the equilibrium level
- Page 357 and 358:
lpZ batu D;k gS \ buds izdkj o mi;k
- Page 359 and 360:
xk¡/kh ds fopkjksa ds lzksrksa dk
- Page 361 and 362:
fo'o dks"k dk vFkZ ,oa mi;ksfxrk cr
- Page 363 and 364:
1. Bargaining theory of wages is be
- Page 365 and 366:
1. Write in detail about drug abuse
- Page 367 and 368:
A CFE-02 C.F.E. EXAMINATION, June,
- Page 369 and 370:
(iii) 'had' in 'We'd finished' (iv)
- Page 371 and 372:
(i) Business started with cash 1,75
- Page 373 and 374:
fuEufyf[kr ij laf{kIr fVIif.k;k¡ f
- Page 375 and 376:
A MCOM-07 M.Com. (Final) EXAMINATIO
- Page 377 and 378:
A BT-10 B.Sc. (Part III) EXAMINATIO
- Page 379 and 380:
(ii) MsyusV dh xfrfof/k;k¡ (iii) b
- Page 381 and 382:
A MAED-08 M.A. (Final) EXAMINATION,
- Page 383 and 384:
A COS-02 C.O.S. EXAMINATION, June,
- Page 385 and 386:
6. Describe any two : (i) Synovial
- Page 387 and 388:
1. Examine the Centre-State relatio
- Page 389 and 390:
1. Describe Balban's concept of kin
- Page 391 and 392:
{ks=h; vkS|ksfxd fodkl ds fØ;kUo;u
- Page 393 and 394:
i;kZoj.k dk laf{kIr ifjp; fyf[k;s ,
- Page 395 and 396:
cUn rkys dh Hkk¡fr dj fn;k tk, ftl
- Page 397 and 398:
A HI-01 B.A. (First Year) EXAMINATI
- Page 399 and 400:
A DSPR-03 D.S.P.R. EXAMINATION, Jun
- Page 401 and 402:
(ii) Innovations in teaching learni
- Page 403 and 404:
A ESO-01 B.A. EXAMINATION, June, 20
- Page 405 and 406:
3. What do you mean by system appro
- Page 407 and 408:
izoklh Jfed dh D;k leL;k,¡ gSa \ b
- Page 409 and 410:
lalnh; jktuhfrd laLFkkvksa dh fo'ks
- Page 411 and 412:
lekpkj izs"k.k esa jsfM;ks dh izHkk
- Page 413 and 414:
(iv) 8"................. (v) 11"...
- Page 415 and 416:
Or Write notes on the following : (
- Page 417 and 418:
Scholar No. : Roll No. : Centre Sea
- Page 419 and 420:
(iv) Statistics of income tax .....
- Page 421 and 422:
Section-B [k.M-c (Decimal Classific
- Page 423 and 424:
(ii) The question paper is divided
- Page 425 and 426:
2. Classify any five titles of the
- Page 427 and 428:
A B.Ed.-10 B.Ed. EXAMINATION, June,
- Page 429 and 430:
5. If you have to teach a lesson 'K
- Page 431 and 432:
O;kolkf;d f'k{kk dk vFkZ ,oa {ks= c
- Page 433 and 434:
5. Discuss the role and importance
- Page 435 and 436:
4. Cost Accounting can usefully be
- Page 437 and 438:
2. "Teach grammar as and for genuin
- Page 439 and 440:
(b) Briefly explain 8085 program st
- Page 441 and 442:
A MEC/MAEC-09 M.A. (Final) EXAMINAT
- Page 443 and 444:
A MASO-08 M.A. (Final) EXAMINATION,
- Page 445 and 446:
(ii) Motivation (iii) POSDCORB (iv)
- Page 447 and 448:
10. Explain control system. What ar
- Page 449 and 450:
A MAPA-08 M.A. (Final) EXAMINATION,
- Page 451 and 452:
A MPS/MAPS-09 M.A. (Final) EXAMINAT
- Page 453 and 454:
5. Discuss the causes of the Santha
- Page 455 and 456:
A ECO-04 B.Com. EXAMINATION, June,
- Page 457 and 458:
3. Discuss the tasks and responsibi
- Page 459 and 460:
lksfM;e DyksjkbM dh fØLVy lajpuk d
- Page 461 and 462:
A DLIS-03 Diploma in Library and In
- Page 463 and 464:
Title 6 : NICHOLAS RUBAKIN AND PSYC
- Page 465 and 466:
5. Describe various types of senten
- Page 467 and 468:
5. Examine challenges of Tourism ma
- Page 469 and 470:
5. Write a short note on Planning o
- Page 471 and 472:
3. Explain the potential energy cur
- Page 473 and 474:
5. Discuss export procedure. 6. Exa
- Page 475 and 476:
2. Describe structure and functions
- Page 477 and 478:
3. Evaluate the cultural achievemen
- Page 479 and 480:
2. Derive classical wave equation a
- Page 481 and 482:
1. Define the term of Public Admini
- Page 483 and 484:
1. "Modern Political Science is the
- Page 485 and 486:
vjs rqe D;k ns'k dk m)kj djksxs ! i
- Page 487 and 488:
(ii) The question paper is divided
- Page 489 and 490:
2. Classify any five of the followi
- Page 491 and 492:
Scholar No. : Roll No. : Centre Sea
- Page 493 and 494:
(v) Russian investment in India ...
- Page 495 and 496:
Section-B [k.M-c (Dewey Decimal Cla
- Page 497 and 498:
(ii) The question paper is divided
- Page 499 and 500:
2. Classify any five of the followi
- Page 501 and 502:
7. Write a short note on 'Dandi Yat
- Page 503 and 504:
6. Explain the objects of Office Fo
- Page 505 and 506:
A MCOM-08 M.Com. (Final) EXAMINATIO
- Page 507 and 508:
Overhead 1,200 1,350 1,440 Input un
- Page 509 and 510:
8. From the following, find out : (
- Page 511 and 512:
Li'kkZuqdwyk bo lw;ZdkUrk] LrnU;rst
- Page 513 and 514:
vki eksVj dSls pyk ldsaxs \ t#j ,Dl
- Page 515 and 516:
dh
- Page 517 and 518:
6. Describe the nature of land-owne
- Page 519 and 520:
A B.Ed.-13 B.Ed. EXAMINATION, June,
- Page 521 and 522:
5. Describe the utility of language
- Page 523 and 524:
ek/;fed Lrj ij jlk;u foKku f'k{k.k
- Page 525 and 526:
Other information : Class No. : Xm4
- Page 527 and 528:
Pages : XI, 295 Size : 23 cm H.T.P.
- Page 529 and 530:
Other information : Call No. : Am3,
- Page 531 and 532:
Other information : Call No. : 153
- Page 533 and 534:
;fn V fdlh {ks= F ij lfn'k lef"V vk
- Page 535 and 536:
(i) W1 , W2 W W1 W2 W (ii) a, w F
- Page 537 and 538:
vFkok miU;kl jS rÙoka jS vk/kkj ek
- Page 539 and 540:
(iii) nf{k.kk (iv) czãk 7. /ofu if
- Page 541 and 542:
(iii) DokdZ ,Dlizsl (iv) CykWx (v)
- Page 543 and 544:
A MP-101 M.B.A. (First Semester) EX
- Page 545 and 546:
4. Explain the Growth curve with th
- Page 547 and 548:
5. Explain 'New Product'. Discuss t
- Page 549 and 550:
A MA/MSc-GE-01 M.A./M.Sc. (Previous
- Page 551 and 552:
A MAED-11 M.A. (Education) (Final)
- Page 553 and 554:
A MAED-12 M.A. (Final) EXAMINATION,
- Page 555 and 556:
10. Write short notes on any two of
- Page 557 and 558:
10. Discuss various geo-economic pr
- Page 559 and 560:
fuEufyf[kr esa ls fdUgha nks ij laf
- Page 561 and 562:
A MARJ-03 ,e- ,- ¼iwokZ)Z½ ijh{kk
- Page 563 and 564:
6. Describe powers of the Supreme C
- Page 565 and 566:
,dkf/kdkjkRed izfr;ksfxrk dk egÙo
- Page 567 and 568:
A B.Ed.-15 (New) B.Ed. EXAMINATION,
- Page 569 and 570:
4. Write a basic conceptual scheme
- Page 571 and 572:
1. Find out the median from the fol
- Page 573 and 574:
13 16 14 2 15 2 3. Calculate Karl P
- Page 575 and 576:
f'k{kk esa ^MkW- ch-,l- Cywe* us m
- Page 577 and 578:
A B.Ed.-20 B.Ed. EXAMINATION, June,
- Page 579 and 580:
fu;fer ikB;kstuk D;k gS \ ,d f'k{kd
- Page 581 and 582:
lizlax O;k[;k dhft;s % vFkok (i) fg
- Page 583 and 584:
fuEufyf[kr ij laf{kIr fVIif.k;k¡ f
- Page 585 and 586:
{kh.k ;qXeu dh fLFkfr esa ;qfXer nk
- Page 587 and 588:
fl) dhft;s fd izR;sd vlhfer ifjc) l
- Page 589 and 590:
fl) dhft;s fd Qyu f bx , yg fb0 , 0
- Page 591 and 592:
A EHD-02 B.A. EXAMINATION, June, 20
- Page 593 and 594:
;g xhr Hkqokyh dh gS gok gqtwj ;g x
- Page 595 and 596:
,gok opu |ke.kka cksyb] fcgwa dfj i
- Page 597 and 598:
ckckslk jh HkksGko.k lwa iwaN idM+G
- Page 599 and 600:
vFkok ukukukekJ;ksRiUua fu?k.Vqfuxe
- Page 601 and 602:
vFkok twuS jktLFkkuh x| jk U;kjk&U;
- Page 603 and 604:
9. (a) Using Biot-Savart's law deri
- Page 605 and 606:
ykIykfl;u ladkjd ds dkrhZ;] csyuh;
- Page 607 and 608:
2. (a) Let R be a relation on set o
- Page 609 and 610:
fuEufyf[kr dks ifjHkkf"kr dhft;s %
- Page 611 and 612:
A MP-202 M.B.A. (II Semester) EXAMI
- Page 613 and 614:
Find out : (i) P.V. ratio (ii) Brea
- Page 615 and 616:
9. Write short notes on any two of
- Page 617 and 618:
A MASA-04 M.A. (Previous) EXAMINATI
- Page 619 and 620:
3. Hkkjrh;n'kZukfuA 4. jhfrjkRek dk
- Page 621 and 622:
ukxfjd 'kkL= f'k{k.k esa ikB~;Øe f
- Page 623 and 624:
A B.Ed.-22 (New) B.Ed. EXAMINATION,
- Page 625 and 626:
Part-B Hkkx-c Attempt any four ques
- Page 627 and 628:
[k.M&l bl [k.M ls dqy rhu iz'uksa d
- Page 629 and 630:
l [k.M esa nks y?kwÙkjkRed iz'u gS
- Page 631 and 632:
(b) Evaluate : 2 5 O eku Kkr dhft;
- Page 633 and 634:
x f (x) 120 49225 125 48316 130 472
- Page 635 and 636:
ckj ekaxlh] b.k [kkrj mRrj bZ dksuh
- Page 637 and 638:
2. ^oØksfDr dkO; thfore~* jh foLrk
- Page 639 and 640:
A MARJ-08 M.A. (Final) EXAMINATION,
- Page 641 and 642:
A MARJ-04 M.A. (Previous) EXAMINATI
- Page 643 and 644:
¼[k½ jkefr;k er rksM+ ¼x½ tkxrh
- Page 645 and 646:
2 3 v/kZ?ku ijoy; ay x ds 'kh"kZ l
- Page 647 and 648:
(b) Find the point of inflexion of
- Page 649 and 650:
act along OA, OB, OC and are in equ
- Page 651 and 652:
(b) The ends of an uniform chain sl
- Page 653 and 654:
A MP-203 M.B.A. (Second Semester) E
- Page 655 and 656:
A GE-08 B.A./B.Sc. (Third Year) EXA
- Page 657 and 658:
9. Give Botanical name, family and
- Page 659 and 660:
A BO-10 B.Sc. (Part III) EXAMINATIO
- Page 661 and 662:
7. Write notes on the following : (
- Page 663 and 664:
xk¡/khth }kjk izfrikfnr lR;kxzg ds
- Page 665 and 666:
2. Describe briefly about Bentham a
- Page 667 and 668:
(ii) Factors affecting absorption o
- Page 669 and 670:
2. Describe the molecular structure
- Page 671 and 672:
2. Divide Asia into major soil regi
- Page 673 and 674:
'kkfUr LFkkiuk dk vFkZ crkrs gq, 'k
- Page 675 and 676:
prove that G' H if and only if H i
- Page 677 and 678:
10. Divide world into major resourc
- Page 679 and 680:
A ED-03 B.A. (Second Year) EXAMINAT
- Page 681 and 682:
A PH-10 B.Sc. (Part III) EXAMINATIO
- Page 683 and 684:
,d ijek.kqd js[kh; Ükà[kyk ds fy,
- Page 685 and 686:
[k.M-II 2. [;kr lkfgR; jkS vjFk crk
- Page 687 and 688:
jksGlh [kGnGk¡ p[kS jkra cjhA dGk;
- Page 689 and 690:
Hkkjr esa lekpkj lfefr;ksa ds mn~Hk
- Page 691 and 692:
(b) Discuss distribution of energy
- Page 693 and 694:
(ii) 'kkfUr f'k{kk dk vkSfpR; (iii)
- Page 695 and 696:
(iii) 'kfä'kkyh dh vfgalk (iv) /kk
- Page 697 and 698:
fuEufyf[kr esa ls fdUgha nks ij 250
- Page 699 and 700:
A M.A./M.Sc.-MT-02 M.A./M.Sc. (Prev
- Page 701 and 702:
(b) Show that a second countable sp
- Page 703 and 704:
2. (a) Solve : c h 2 2 2 y z x dx
- Page 705 and 706:
A M.A./M.Sc.-MT-04 M.A./M.Sc. (Prev
- Page 707 and 708:
7. (a) Show that at a point on a su
- Page 709 and 710:
3. An ellipse of axes a and b and a
- Page 711 and 712:
Then prove that N/M is a normed lin
- Page 713 and 714:
Mr. Ram to whom 160 shares were all
- Page 715 and 716:
Payments Rs. By salaries 15,120 By
- Page 717 and 718:
A MT-08 B.A./B.Sc. (Part III) EXAMI
- Page 719 and 720:
10. (a) Prove that fuEu Qyu dk vuUr
- Page 721 and 722:
z = 0 and determine the region of c
- Page 723 and 724:
dk dsUnz Kkr dhft;s ,oa bl dsUnz ls
- Page 725 and 726:
(b) Find the equation of right circ
- Page 727 and 728:
(iii) Describe the structure of end
- Page 729 and 730:
thok.kq esa jkbckslkse fdl izdkj dk
- Page 731 and 732:
vPNs izsl izcU/ku dk mYys[k djrs gq
- Page 733 and 734:
fodsUnzhÑr ;kstuk ij xk¡/kh ds fo
- Page 735 and 736:
Hkwnku ds fy, fouksck Hkkos ds ;ksx
- Page 737 and 738:
2. Gandhi and Satyagraha. xk¡/kh ,
- Page 739 and 740:
4. Discuss the important theories o
- Page 741 and 742:
4. Compare and contrast Addison and
- Page 743 and 744:
A MPSA-01 M.Phil EXAMINATION, June,
- Page 745 and 746:
A MPSA-02 M.Phil EXAMINATION, June,
- Page 747 and 748:
A MPSA-03 M.Phil EXAMINATION, June,
- Page 749 and 750:
(ii) gfj% oSdq.Be/;kLrsA (iii) jkes
- Page 751 and 752:
customers service satisfaction with
- Page 753 and 754:
^^u dsoy ikuh dh i;kZIr ek=k cfYd m
- Page 755 and 756:
lkoZtfud iqLrdky;ksa dh cnyrh Hkwfe
- Page 757 and 758:
A MPHD-02 M.Phil EXAMINATION, June,
- Page 759 and 760:
A MPHD-01 M.Phil EXAMINATION, June,