final.pdf
final.pdf
final.pdf
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
Name: ___________________________________________<br />
Bi/Ch 110 Final Exam<br />
The FIRST EXAM QUESTION is to write the name, the one letter code, the three letter<br />
code, and the structure of the twenty amino acids. This portion of the test is CLOSED<br />
BOOK.<br />
Only the Stryer “Biochemistry” textbook, your lecture notes, this year’s (Fall 2012)<br />
homework sets solutions, and materials on this year’s Bi110 course website (or that were<br />
emailed to the class) can be used for the remaining open book section of the test. NO<br />
INTERNET.<br />
You may use calculators, graphing programs such as Excel, and the standard wordprocessing<br />
software if you are going to type your answers. You may not use computers<br />
for any other resources.<br />
The time limit on the exam is 6 hours not counting the time writing the twenty amino<br />
acids. You may take the exam in two sittings as long as you don’t go over 6 hours for<br />
the whole exam. You are NOT allowed to study during your break.<br />
Write your name on every page submitted and number each page as well. Do not put<br />
answers to different questions on the same piece of paper or you will lose points.<br />
You may e-mail the TAs for clarifications on the questions, but you may not ask about<br />
the validity of your answers. Your e-mails must be sent at least 12 hours prior to the<br />
deadline. Please address the emails to all of the TAs for the <strong>final</strong> exam.<br />
If you cannot read a figure on your printed exam, please refer to the electronic version of<br />
the exam.<br />
The strict due date is 4:30pm Friday, Dec. 14 th at Margot Hoyt’s office, 148 Braun.<br />
Late exams will not be accepted. Extensions will not be granted.
Name: ___________________________________________<br />
Open the exam to begin the CLOSED BOOK portion.
Problem 1: Amino Acids (20 points)<br />
(CLOSED BOOK, NOTES, AND COURSE WEBSITE)<br />
Name: ___________________________________________<br />
Draw the 20 amino acids by hand. Do not worry about stereochemistry.
Problem 2: Enzyme Kinetics<br />
Name: ___________________________________________<br />
Glyphosate is the herbicide also known as Roundup. It is a broad-spectrum herbicide that<br />
inhibits the enzyme 5-enolpyruvylshikimate-3-phosphate synthase (EPSPS), which catalyzes<br />
the reaction of shikimate-3-phosphate (S3P) and phosphoenolpyruvate (PEP) to form 5enolpyruvyl-shikimate-3-phosphate<br />
(ESP):<br />
PEP + S3P ESP + phosphate<br />
ESP is dephosphorylated to chorismate, a precursor for the aromatic amino acids<br />
phenylalanine, tyrosine, and tryptophan, which plants must synthesize in order to survive<br />
(animals get these amino acids from their diet).<br />
a. (10 points) The following two plots show the effect of glyphosate on EPSPS activity<br />
when measuring PEP. What type of inhibitor is glyphosate, based on this experiment?
Problem 2, page 2<br />
Name: ___________________________________________<br />
b. (10 points) The following plot shows the effect of glyphosate on EPSPS activity when<br />
measuring S3P. What type of inhibitor is glyphosate, based on this experiment?<br />
c. (10 points) Based on your above two answers, propose the mechanism or mechanisms<br />
by which glyphosate inhibits EPSPS. Answer in no more than four sentences or by<br />
drawing a simple picture with labels. You do not need to know the intricacies of the<br />
molecular pathways; just describe how you think glyphosate, EPSPS, PEP, and S3P<br />
interact with each other.
Problem 3: TCA Cycle<br />
Name: ___________________________________________<br />
Fluoroacetate is a popular and potent rat poison that is found in the leaves of certain<br />
poisonous plants. Sodium fluoroacetate is metabolized into fluoroacetyl-CoA by the enzyme<br />
acetate thiokinase and then is incorporated into fluorocitrate as shown.<br />
A scientist studying the effect of sodium fluoroacetate on mammalian muscle cells found a<br />
decrease in the rate of glycolysis and in the amount of citric acid cycle (TCA) intermediates.<br />
However, citrate levels increased several fold.<br />
a. (10 points) What step in the TCA cycle appears to be blocked? Why do citrate levels<br />
increase dramatically, while the levels of the other TCA intermediates decrease?
Problem 3, page 2<br />
Name: ___________________________________________<br />
b. (10 points) Aconitase catalyzes a reaction with fluorocitrate that yields the following<br />
molecule:<br />
* Represents a 14 C label originating in the fluoroacetate, as shown above. (Hint: Recall the<br />
prochirality of citrate in the TCA cycle).<br />
Draw the aconitase-catalyzed reaction that would generate this product in the presence of<br />
fluorocitrate.<br />
c. (10 points) How does this product cause the effects on the TCA cycle described above<br />
(molecular mechanism, stating the specific molecules involved)?
Problem 4: ATCase and Cooperativity<br />
Name: ___________________________________________<br />
Aspartate transcarbamoylase (ATCase) is an enzyme at the beginning of the pyrimidine<br />
biosynthesis pathway, which catalyzes the formation of carbamoyl aspartate from<br />
carbamoylphosphate and aspartate.<br />
a. (5 points) Does the ATCase (control curve shown below) follow typical Michaelis-Menten<br />
behavior? If not, what type of enzyme might this be?
Problem 4, page 2<br />
Name: ___________________________________________<br />
b. (5 points) Low millimolar concentrations of CTP and ATP affect the relative reaction rate<br />
of ATCase. Which curve (either A or B) would you expect to see in the presence of<br />
CTP? How about ATP? Explain briefly (3 sentence maximum).<br />
c. (15 points) Maleate binds to the same site in ATCase as the substrate aspartate<br />
preventing the concomitant binding of aspartate. The KI of maleate is significantly<br />
smaller than the Km of aspartate. Upon heating and renaturation, the enzyme has<br />
activity that is inhibited by maleate as shown in the figure below. However, the native<br />
enzyme is activated at low concentrations of maleate and inhibited at high<br />
concentrations of maleate. Explain this result.
Problem 4, page 3<br />
Name: ___________________________________________<br />
The data shown below were gathered at a constant aspartate concentration of 10-3 M<br />
and constant carbamyl phosphate concentration of 3.6 x 10-3 M:<br />
d. (10 points) Compare the effect of maleate on ATCase with the effect of carbon monoxide<br />
on oxygen delivery by hemoglobin.<br />
e. (5 points) Demonstrate why the Hill coefficient, n, can be equal to or less than, but never<br />
more than, the number of substrate binding subunits in an allosteric cooperative protein.<br />
E.g. for hemoglobin, n ≤ 4.
Problem 5: ATP Synthase<br />
Name: ___________________________________________<br />
In 1961 Peter Mitchell proposed that a proton gradient across the mitochondrial membrane is<br />
used to create ATP. The inside of mitochondria has a pH 1.4 units higher than the outside, and<br />
the voltage difference is 0.14 V with the outside being positive.<br />
a. (15 points) Using this information and the equation on page 385 of your book, calculate<br />
the free energy associated with pumping 1 mole of protons from the mitochondrial matrix<br />
to the cytosol.<br />
b. (10 points) Considering the free energy associated with synthesis of ATP from ADP and<br />
phosphate is 30.5 kJ/mol, what would you expect to be the minimum number of protons<br />
transferred across the membrane per ATP molecule produced?<br />
c. (10 points) How many protons are actually required for each ATP molecule? What<br />
percentage efficiency does this correspond to?
Problem 6: Mechanism (20 points)<br />
Name: ___________________________________________<br />
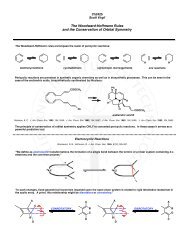
Propose a mechanism for the conversion of Δ 3 – friedelin (1) to Δ 13(18) – oleanene (2).
Problem 7: pH Optimum of an Enzyme (20 points)<br />
Name: ___________________________________________<br />
The active site of lysozyme contains two amino acid residues essential for catalysis: Glu 35 and<br />
Asp 52 . The pKa values of the carboxyl side chains of these residues are 5.9 and 4.5,<br />
respectively. What is the ionization state (protonated or deprotonated) of each residue at pH<br />
5.2, the pH optimum of lysozyme? How can the ionization states of these residues explain the<br />
pH-activity profile of lysozyme shown below?
Problem 8: Protein Folding and Structure<br />
Name: ___________________________________________<br />
a. (10 points) Explain why β-sheets are less likely to form than α-helices during the earliest<br />
stages of protein folding.<br />
b. (25 points) Indicate the probable effects of the following mutational changes on the<br />
structure of a protein. Explain your reasoning.<br />
i. Changing a Leu to a Phe<br />
ii. Changing a Lys to a Gly<br />
iii. Changing a Val to a Thr<br />
iv. Changing a Gly to an Ala
Problem 8, page 2<br />
v. Changing a Met to a Pro<br />
Name: ___________________________________________<br />
c. (10 points) Explain why Trp rings are usually completely immobile in proteins that have<br />
rapidly flipping Phe and Tyr rings.<br />
d. The unfolding of the α helix of a polypeptide to a randomly coiled is accompanied by a<br />
large decrease in a property called specific rotation, a measure of a solution’s capacity<br />
to rotate circularly polarized light. Polyglutamate, a polypeptide made up of only L-Glu<br />
residues, has the α-helical conformation at pH 3. When the pH is raised to 7, there is a<br />
large decrease in the specific rotation of the solution. Similarly polylysine (L-Lys<br />
residues) is an α helix at pH 10, but when the pH is lowered to 7 the specific rotation<br />
also decreases, as shown by the following graph:
Problem 8, page 3<br />
Name: ___________________________________________<br />
i. (10 points) What is the explanation for the effect of the pH changes on the<br />
conformations of poy(Glu) and poly(Lys)?<br />
ii. (10 points) Why does the transition occur over such a narrow range of pH?
Problem 9: Alcohol Dehydrogenase<br />
Name: ___________________________________________<br />
a. (10 points) Write down the reaction catalyzed by alcohol dehydrogenase when ethanol is<br />
the substrate. Be sure to label where the hydrogen in NADH goes.<br />
b. (10 points) What’s the function of Zn 2+ in alcohol dehydrogenase?<br />
c. It is found that if monodeuterated alcohol (CH3CDHOH) is used as the substrate, there<br />
can be two different outcomes of this reaction. In one outcome, NADD and CH3CHO are<br />
generated and in the other outcome, NADH and CH3CDO are generated. If these two<br />
outcomes don’t appear randomly,<br />
i. (5 points) What can be the causes of the difference between these two<br />
outcomes?
Problem 9, page 2<br />
Name: ___________________________________________<br />
ii. (5 points) In what condition can only one outcome happen (using the same<br />
enzyme)?<br />
d. (15 points) The consumption of ethanol, especially after periods of strenuous activity or<br />
after not eating for several hours, results in a deficiency of glucose in the blood, a<br />
condition known as hypoglycemia. The first step in the metabolism of ethanol by the<br />
liver is oxidation to acetaldehyde, catalyzed by liver alcohol dehydrogenase:<br />
CH3CH2OH + NAD + CH3CHO + NADH + H +<br />
Explain how this reaction inhibits the transformation of lactate to pyruvate. Why does<br />
this lead to hypoglycemia?
Problem 10: GroEL/ES (25 points)<br />
Name: ___________________________________________<br />
The GroEL/ES cycle diagrammed below only circulates in the clockwise direction. Explain the<br />
basis for this irreversibility in terms of the sequence of structural and binding changes in the<br />
GroEL/ES system. Write on the back of the page or a different page if you need more room.
Problem 11: The Pasteur Effect<br />
Name: ___________________________________________<br />
When oxygen is added to an anaerobic suspension of cells consuming glucose at a high rate,<br />
the rate of glucose consumption declines greatly as the oxygen is used up, and accumulation of<br />
lactate ceases. This effect, first observed by Louis Pasteur in the 1860s, is characteristic of<br />
most cells capable of both aerobic and anaerobic glucose catabolism.<br />
a. (10 points) Why does the accumulation of lactate cease after oxygen is added?<br />
b. (10 points) Why does the presence of oxygen decrease the rate of glucose<br />
consumption?<br />
c. (10 points) How does the onset of oxygen consumption slow down the rate of<br />
glucose consumption? Explain in terms of specific enzymes.