Opioids 1: Opiates - College of American Pathologists
Opioids 1: Opiates - College of American Pathologists
Opioids 1: Opiates - College of American Pathologists
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
Chapter 17<br />
<strong>Opioids</strong> 1: <strong>Opiates</strong><br />
Michael G. Bissell, MD, PhD, MPH<br />
Michael A. Peat, PhD<br />
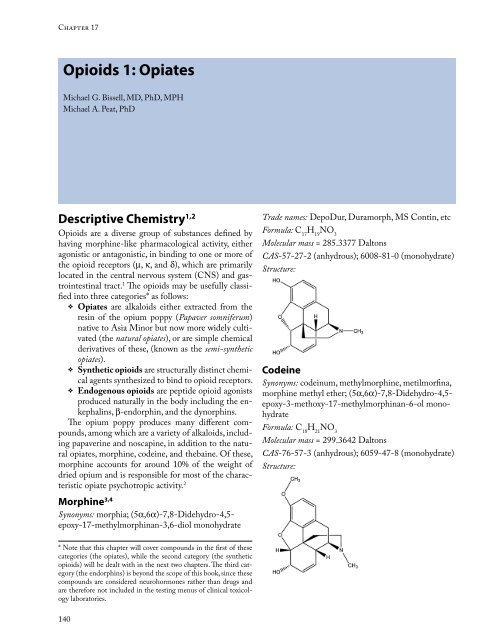
Descriptive Chemistry 1,2<br />
<strong>Opioids</strong> are a diverse group <strong>of</strong> substances defined by<br />
having morphine-like pharmacological activity, either<br />
agonistic or antagonistic, in binding to one or more <strong>of</strong><br />
the opioid receptors (m, k, and d), which are primarily<br />
located in the central nervous system (CNS) and gastrointestinal<br />
tract. 1 The opioids may be usefully classified<br />
into three categories* as follows:<br />
v <strong>Opiates</strong> are alkaloids either extracted from the<br />
resin <strong>of</strong> the opium poppy (Papaver somniferum)<br />
native to Asia Minor but now more widely cultivated<br />
(the natural opiates), or are simple chemical<br />
derivatives <strong>of</strong> these, (known as the semi-synthetic<br />
opiates).<br />
v Synthetic opioids are structurally distinct chemical<br />
agents synthesized to bind to opioid receptors.<br />
v Endogenous opioids are peptide opioid agonists<br />
produced naturally in the body including the enkephalins,<br />
b-endorphin, and the dynorphins.<br />
The opium poppy produces many different compounds,<br />
among which are a variety <strong>of</strong> alkaloids, including<br />
papaverine and noscapine, in addition to the natural<br />
opiates, morphine, codeine, and thebaine. Of these,<br />
morphine accounts for around 10% <strong>of</strong> the weight <strong>of</strong><br />
dried opium and is responsible for most <strong>of</strong> the characteristic<br />
opiate psychotropic activity. 2<br />
Morphine 3,4<br />
Synonyms: morphia; (5a,6a)-7,8-Didehydro-4,5epoxy-17-methylmorphinan-3,6-diol<br />
monohydrate<br />
* Note that this chapter will cover compounds in the first <strong>of</strong> these<br />
categories (the opiates), while the second category (the synthetic<br />
opioids) will be dealt with in the next two chapters. The third category<br />
(the endorphins) is beyond the scope <strong>of</strong> this book, since these<br />
compounds are considered neurohormones rather than drugs and<br />
are therefore not included in the testing menus <strong>of</strong> clinical toxicology<br />
laboratories.<br />
140<br />
Trade names: DepoDur, Duramorph, MS Contin, etc<br />
Formula: C H NO 17 19 3<br />
Molecular mass = 285.3377 Daltons<br />
CAS-57-27-2 (anhydrous); 6008-81-0 (monohydrate)<br />
Structure:<br />
Codeine<br />
Synonyms: codeinum, methylmorphine, metilmorfina,<br />
morphine methyl ether; (5a,6a)-7,8-Didehydro-4,5epoxy-3-methoxy-17-methylmorphinan-6-olmonohydrate<br />
Formula: C H NO 18 21 3<br />
Molecular mass = 299.3642 Daltons<br />
CAS-76-57-3 (anhydrous); 6059-47-8 (monohydrate)<br />
Structure:
Thebaine<br />
Synonyms: paramorphine; (5a)-6,7,8-Tetradehydro-4,5-epoxy-3-methoxy-17-methylmorphinan<br />
Formula: C H NO 19 21 3<br />
Molecular mass = 311.3749 Daltons<br />
CAS-115-37-7<br />
Structure:<br />
The semi-synthetic opiates, including heroin, hydromorphone,<br />
hydrocodone, oxymorphone, and oxycodone,<br />
are simple chemical modifications <strong>of</strong> morphine<br />
or thebaine.<br />
Heroin<br />
Synonyms: acetomorphine, diacetylmorphine, diamorphine;(5a,6a)-7,8-Didehydro-4,5-epoxy-17-methylmorphinan-3,6-d<br />
diacetate (ester)<br />
Formula: C H NO 21 23 5<br />
Molecular mass = 369.4110 Daltons<br />
CAS-561-27-3<br />
Structure:<br />
Hydromorphone<br />
Synonyms: dihydromorphinone, dimorphone; 4,5-epoxy-3-hydroxy-17-methylmorphinan-6-one<br />
Trade names: Dilaudid, Palladone<br />
Formula: C H NO 17 19 3<br />
Molecular mass = 285.3377 Daltons<br />
CAS-466-99-9<br />
Structure:<br />
<strong>Opioids</strong> 1: <strong>Opiates</strong> | 17<br />
Hydrocodone<br />
Synonyms: dihydrocodeinone; 4,5-epoxy-3-methoxy-<br />
17-methylmorphinan-6-one<br />
Trade names: Vicodin, Panacet, etc<br />
Formula: C H NO 18 21 3<br />
Molecular mass = 299.3642 Daltons<br />
CAS-125-29-1<br />
Structure:<br />
Oxymorphone<br />
Synonyms: 7,8-Dihydro-14-hydroxymorphinone;<br />
oximorphone; oxydimorphone; (5a)-4,5-epoxy-3,14dihydroxy-17-methylmorphinan-6-one<br />
Trade names: Numorphan, Opana<br />
Formula: C H NO 17 19 4<br />
Molecular mass = 301.3371 Daltons<br />
CAS-76-41-5<br />
Structure:<br />
141
III A | The Toxicology Laboratory’s Test Menu: Abused Substances<br />
Oxycodone<br />
Synonyms: 7,8-Dihydro-14-hydroxycodeinone; dihydrone;<br />
oxycone; (5a)- 4,5-epoxy-14-hydroxy-3-methoxy-17-methylmorphinan-6-one<br />
Trade names: Oxycontin, Percocet, Percodan, etc<br />
Formula: C H NO 18 21 4<br />
Molecular mass = 315.3636<br />
CAS-76-42-6<br />
Structure:<br />
Origin 1,2,5<br />
Opium, the viscous fluid within the unripe capsule <strong>of</strong><br />
the flowering head <strong>of</strong> the opium poppy, is the source<br />
<strong>of</strong> the naturally occurring opiates. Opium was originally<br />
administered by smoking—and still is in southeast<br />
Asian communities around the world. Tincture <strong>of</strong><br />
opium and paregoric are traditional oral pharmaceutical<br />
preparations <strong>of</strong> opium. Morphine and codeine, the<br />
natural opiates in use as drugs today, are obtained, legally<br />
or illegally, in commercially purified oral or IV<br />
pharmaceutical preparations. This is also true <strong>of</strong> the<br />
semi-synthetic opiates, the morphine derivatives hydromorphone<br />
(Dilaudid) and hydrocodone (Vicodin,<br />
etc), and the thebaine derivatives oxycodone (Oxycontin,<br />
etc) and oxymorphone (Numorphan, Opana), all <strong>of</strong><br />
which have legitimate places in the pharmacopeia. The<br />
exception among this class <strong>of</strong> drugs is heroin, which,<br />
although available legally for treatment <strong>of</strong> terminal<br />
cancer pain in other parts <strong>of</strong> the world (“Brompton<br />
cocktail”), is a Schedule I substance (ie, available only<br />
illicitly) in the US.<br />
Heroin is made by extracting morphine from opium<br />
and acetylating it at the two phenolic groups. Supplied<br />
in varying degrees <strong>of</strong> purity (2% to 6%) on the street<br />
as a white to brownish powder, heroin is nearly always<br />
“cut” with other substances as it works its way through<br />
the chain <strong>of</strong> distribution, being commonly adulterated<br />
with other depressant drugs like benzodiazepines or<br />
other opioids, 2 diluted with neutral powders like sugar,<br />
and sold in packets containing 3 to 16 mg heroin each.<br />
The chemical form <strong>of</strong> the heroin itself depends to some<br />
142<br />
extent on where the opium was originally harvested<br />
and processed, the two main opium-growing regions<br />
being in central Asia (Afghanistan, Pakistan, Iran),<br />
known as the “Golden Crescent,” and in southeast Asia<br />
(Laos, Myanmar, Thailand), known as the “Golden Triangle.”<br />
Heroin from the former region is most typically<br />
supplied as the hydrochloride salt, which is soluble in<br />
cold water, whereas that from the latter region may be<br />
in the form <strong>of</strong> the free base, which must be heated in<br />
an acidic solution before injection. Known by a number<br />
<strong>of</strong> slang synonyms such as “H,” “smack,” “junk,” “horse,”<br />
and “skag,” heroin is among the most widely abused<br />
opioids because <strong>of</strong> its combination <strong>of</strong> being lipophilic<br />
and water soluble, making it bioavailable and potent,<br />
facilitating rapid achievement <strong>of</strong> psychoactive concentrations<br />
in the brain. Although the powder can be administered<br />
by inhalation <strong>of</strong> the vapor (“skagging”), by<br />
nasal insufflation (“snorting”), or by smoking in “reefers,”<br />
it is most commonly injected intravenously or subcutaneously<br />
in doses <strong>of</strong> up to 200 mg per day.<br />
Pharmacology<br />
Type <strong>of</strong> Agent 1,6<br />
The opioids in general are classified as narcotic analgesics<br />
and CNS depressants, although they have a variety<br />
<strong>of</strong> therapeutic effects including analgesia, antitussive<br />
effects, and decreasing gastrointestinal motility, as well<br />
as side-effects on respiration. The term “narcotic” was<br />
derived from the Greek word for stupor and has been<br />
associated with strong opiate analgesics, but now has<br />
connotations that are more legal than pharmacological.<br />
In 1977, Martin and Sloan 6 proposed the existence <strong>of</strong><br />
multiple opioid receptors, and this has been confirmed<br />
by numerous studies since then. Shortly after the existence<br />
<strong>of</strong> these receptors had been confirmed, three<br />
classes <strong>of</strong> endogenous opioid peptides were identified.<br />
They are encoded by different genes, are expressed in<br />
different neuronal pathways, and have different selectivities.<br />
There are three major classes <strong>of</strong> receptors in the<br />
central nervous system: m, k, and d receptors. There are<br />
a number <strong>of</strong> subtypes within each class. Receptor studies<br />
have revealed differing selectivity pr<strong>of</strong>iles for each<br />
class, and functional studies have also shown differing<br />
functional pr<strong>of</strong>iles. Most <strong>of</strong> the clinically used opioids<br />
are relatively selective for m receptors, but they will interact<br />
with other receptors at higher doses. This is especially<br />
true as doses are increased to overcome tolerance.<br />
Some drugs, particularly mixed agonists/antagonists,<br />
interact with more than one receptor class at usual doses.<br />
When morphine is given systemically, it produces
analgesia through supraspinal m1 receptors. Respiratory<br />
depression and inhibition <strong>of</strong> gastrointestinal motility<br />
are thought to be mediated through m2 receptors. 1<br />
Context <strong>of</strong> Human Use 1,2<br />
Opium has been used and abused for millennia, the<br />
first undisputed reference to the medicinal use <strong>of</strong> poppy<br />
juice being in the writings <strong>of</strong> Theophrastus in the<br />
third century bce. The word “opium” is derived from<br />
the Greek word for “juice.” Opium smoking in a pipe<br />
probably originated in seventeenth century China, and<br />
its abuse became extremely widespread, a problem exacerbated<br />
by the British opium trade and giving rise<br />
to the two nineteenth century Opium Wars. Similarly,<br />
in nineteenth century Europe and America, wide-scale<br />
availability <strong>of</strong> opium and opium derivative medicines,<br />
sold legally over-the-counter for a variety <strong>of</strong> ailments,<br />
led to major problems with addiction and ultimately<br />
to declaring opium an illicit substance. However, the<br />
problem <strong>of</strong> opioid abuse has remained ever since, in<br />
one form or another.<br />
There are more than 20 distinct alkaloids in opium.<br />
In 1806, Serturner isolated morphine from opium,<br />
naming it after Morpheus, the Greek god <strong>of</strong> dreams.<br />
During the nineteenth century, other alkaloids, for<br />
example codeine and papaverine, were isolated. This<br />
resulted in the use <strong>of</strong> the pure alkaloids and their derivatives,<br />
rather than opium. One <strong>of</strong> these derivatives<br />
was diacetylmorphine, originally marketed in 1898 as<br />
a cough medicine and called “Heroin” (from the Greek<br />
word “hero”)—actually felt at the time to be a less addictive<br />
alternative to opium.<br />
The need for effective analgesia grew steadily in<br />
medicine, while at the same time the abuse potential<br />
<strong>of</strong> morphine and derivatives was increasingly being<br />
recognized. These trends during the nineteenth and<br />
early twentieth century led to the search for potent<br />
analgesics free <strong>of</strong> this addictive potential. This resulted<br />
in the introduction <strong>of</strong> the synthetic compounds, such<br />
as meperidine and methadone, around the time <strong>of</strong><br />
World War II. Unfortunately, the problem <strong>of</strong> opioid<br />
abuse has continued unabated, now also involving the<br />
abuse <strong>of</strong> these synthetic compounds, typically obtained<br />
by diversion from legitimate prescription use. Today,<br />
larger numbers <strong>of</strong> individuals than ever before are being<br />
treated for opioid abuse. The first compound with<br />
opioid antagonistic properties was nalorphine, which<br />
was first used in the 1950s to reverse the effects <strong>of</strong> morphine<br />
poisoning. Nalorphine use led to the introduction<br />
<strong>of</strong> purer antagonists, for example naloxone, and<br />
mixed agonists/antagonists, such as pentazocine and<br />
buprenorphine.<br />
<strong>Opioids</strong> 1: <strong>Opiates</strong> | 17<br />
Abuse Potential and Characteristics 1,7-9<br />
The high abuse potential <strong>of</strong> opioids is initially accountable<br />
in terms <strong>of</strong> the “high,” consisting <strong>of</strong> euphoria, followed<br />
by a pleasant warmth, relaxation, and happiness,<br />
without accompanying ataxia. Larger doses produce<br />
dream-like torpor and somnolence. Regular use leads<br />
to definite dependence that is dose and frequency related.<br />
Tolerance to the “high” develops rapidly, and dose<br />
escalation in quest <strong>of</strong> the lost euphoria follows, until a<br />
maximum is reached, <strong>of</strong>ten dictated by practical difficulties<br />
in obtaining increasing quantities <strong>of</strong> the drug.<br />
At this point, tolerance to the positive drug effects becomes<br />
permanent, and the motivation for further drugseeking<br />
shifts from positive to negative reinforcement,<br />
that is, the desire to avoid a withdrawal reaction becomes<br />
primary. Since all opioids share cross-tolerance,<br />
drug substitution (eg, over-the-counter opioids for<br />
heroin) frequently occurs at this point.<br />
Opioid withdrawal includes a range <strong>of</strong> nonfatal but<br />
notoriously unpleasant symptoms generally understandable<br />
in terms <strong>of</strong> a subjective reversal <strong>of</strong> CNS level<br />
<strong>of</strong> arousal from depression to hyperactivity. These may<br />
include initial tearing, runny nose, perspiration, sneezing,<br />
coughing, increased respiratory rate, inability to<br />
sleep, restlessness, and anxiety, followed by chills, goose<br />
bumps, hot flashes, abdominal pain, muscle twitching,<br />
flu-like aching <strong>of</strong> muscles and joints, tremors, sweating,<br />
and headaches. In severe cases, subjects may show fever,<br />
dehydration, elevated blood pressure, rapid heart rate,<br />
and extreme agitation. These symptoms are accompanied<br />
by progressive drug craving, and voluntary efforts<br />
at drug withdrawal frequently result in relapse. Such<br />
attempts, when abruptly undertaken without medical<br />
support, are known as “going cold turkey” (doubtlessly<br />
named for the characteristic piloerection, or “gooseflesh,”<br />
during withdrawal), which typically begins 6<br />
to 12 hours after ceasing heroin, peaking at 36 to 72<br />
hours, and persisting 5 to 10 days, followed by a period<br />
<strong>of</strong> chronic symptoms <strong>of</strong> malaise, fatigue, anxiety,<br />
emotional lability, and depression that may persist for<br />
months. It has been postulated that anti-opioid peptides<br />
may be produced by the brain, which accounts for<br />
the development <strong>of</strong> tolerance in the presence <strong>of</strong> high<br />
levels <strong>of</strong> exogenous opioids. 9 The actions <strong>of</strong> these antiopioids,<br />
unopposed by drug during abstinence, may account<br />
for the symptoms <strong>of</strong> withdrawal. 9<br />
Overdose and Toxicity 10-12<br />
Opiate intoxication and milder degrees <strong>of</strong> overdose<br />
are accompanied by characteristic pupillary constriction<br />
(“pinpoint pupils”), hypotension, bradycardia, hypothermia,<br />
decreased bowel sounds, slowed mentation<br />
143
III A | The Toxicology Laboratory’s Test Menu: Abused Substances<br />
Table 17-1. Toxic Dose 3,10<br />
Drug Estimated Minimum<br />
Lethal Dose (g)<br />
(including slurring <strong>of</strong> speech, confusion, loss <strong>of</strong> alertness,<br />
apathy, and depression), drooping eyelids, and loss<br />
<strong>of</strong> muscle tone, including difficulty maintaining the<br />
head erect (so-called “nodding <strong>of</strong>f ”). There may also be<br />
a degree <strong>of</strong> dose-dependent motor stimulation, but seizures<br />
are rare (more common with synthetic opioids).<br />
More severe overdose leads to coma with respiratory<br />
depression, which may be accompanied by noncardiogenic<br />
pulmonary edema, and which can progress to<br />
sudden death by apnea or gastric aspiration.<br />
Adverse Effects and Complications13,14 Chronic IV heroin use is associated with all the risks <strong>of</strong><br />
self-injection, including peripheral nerve trauma, pulmonary<br />
hypertension, and bloodborne infections when<br />
needle-sharing is practiced.<br />
Chronic opiate abuse more generally is associated<br />
with the following:<br />
Cardiovascular<br />
<strong>Opiates</strong> are associated with hypotension but do not exacerbate<br />
existing hypertension, although some patients<br />
may develop a blood pressure increase during withdrawal.<br />
Respiratory<br />
Since opiates have respiratory depression as a major<br />
side effect, these drugs should be used with care in patients<br />
with any degree <strong>of</strong> respiratory compromise. Heroin<br />
is sometimes smoked or vaporized, with resulting<br />
respiratory symptoms.<br />
Renal<br />
Rhabdomyolysis may be precipitated by intra-arterial<br />
opiates or prolonged unconsciousness during intoxication,<br />
and in turn may precipitate acute renal failure, as<br />
can hypotension associated with IV heroin overdose.<br />
144<br />
Fatal Plasma Concentrations<br />
(µg/mL)<br />
Morphine 120 – 200 mg 0.2 – 2.3 Yes<br />
Codeine 0.5 – 1.0 g 1.0 – 8.8 Yes<br />
Heroin Not reported 0.05 – 3.0 (as free morphine) N/A<br />
Hydromorphone Not reported 0.02 – 1.2 Yes<br />
Hydrocodone 100 mg 0.12 – 1.6 Yes<br />
Oxycodone Not reported 5.0 Yes<br />
Subject to Postmortem<br />
Redistribution<br />
Metabolic<br />
Diabetics’ self-monitoring <strong>of</strong> blood glucose and hypoglycemic<br />
dose adjustment may be disrupted by apathy<br />
and also by vomiting, a recognized side-effect <strong>of</strong> opiates.<br />
Opiate intoxication may be confused with a hypoglycemic<br />
episode by the patient or others.<br />
Immune<br />
There is some evidence that immunoregulatory effects<br />
<strong>of</strong> the endogenous opioids may be disrupted by administration<br />
<strong>of</strong> exogenous opiates.<br />
Reproductive<br />
<strong>Opiates</strong> are not considered to be teratogenic, and although<br />
various perinatal complications have been observed<br />
to be more frequent in heroin-addicted mothers,<br />
it is unclear whether these are attributable specifically<br />
to heroin exposure or to the generally poor health <strong>of</strong><br />
such mothers. Characteristic opiate withdrawal reactions<br />
resembling those in adults may be seen for up to<br />
a week after birth in infants born after third-trimester<br />
opiate exposure. The opiates are expressed in breast<br />
milk, but the resulting levels are insignificant clinically.<br />
Cognitive and behavioral problems have been noted in<br />
preschool-aged children exposed in utero to opiates.<br />
The degree to which these symptoms can be attributed<br />
to drug exposure is unclear.<br />
Toxic Dose<br />
Known toxic dose and concentration range information<br />
is summarized in Table 17-1.<br />
Pharmacokinetics, Metabolism,<br />
and Excretion<br />
Pharmacokinetics<br />
Pharmacokinetic parameters (volume <strong>of</strong> distribution<br />
[Vd] and half-life [t½]) are summarized in Table 17-2.<br />
Drug abusers typically inject heroin or morphine
Table 17-2. Volume <strong>of</strong> Distribution (Vd)<br />
and Half-Life (t ½) 3,10<br />
Drug Vd (L/kg) Plasma t ½ (h)<br />
Morphine 2.0 – 5.0 1.3 – 6.7<br />
Codeine 2.5 – 3.5 1.2 – 4.0<br />
Heroin 25.0 2.0 – 6.0<br />
6.0 – 25.0 (6-MAM)<br />
Hydromorphone 2.0 – 4.0 2.5 – 9.0<br />
Hydrocodone 3.3 – 4.7 3.4 – 8.8<br />
Oxymorphone 2.0 – 4.0 4.0 – 12.0<br />
Oxycodone 1.8 – 3.7 2.0 – 6.0<br />
but take other opiates orally; however, sometimes tablets<br />
or time-release capsules can be crushed and heated<br />
into inhalable or injectable forms. <strong>Opiates</strong> are generally<br />
well absorbed whether taken orally or injected. Injected<br />
opiates result in effects within minutes, whereas after<br />
an oral dose, peak effects typically occur within 2 to<br />
3 hours; however, some slowing <strong>of</strong> absorption <strong>of</strong> oral<br />
opiates may occur due to drug effects on gastrointestinal<br />
tract receptors. Also, the dose form (extended release,<br />
etc) may affect the rate <strong>of</strong> absorption.<br />
Metabolism 1,10,15<br />
The opiates are generally metabolized in the liver by<br />
the cytochrome P450 systems and then conjugated.<br />
Since several <strong>of</strong> the opiates are<br />
metabolized into each other,<br />
the pattern <strong>of</strong> metabolite excretion<br />
requires interpretation<br />
as a whole to determine the<br />
parent drug or drugs involved<br />
in a given case <strong>of</strong> opiate exposure.<br />
The individual drugs are<br />
metabolized as follows 10 :<br />
Heroin is rapidly de-acetylated<br />
to 6-monoactetylmorphine<br />
(6-AM), which in turn<br />
is rapidly hydrolyzed to morphine.<br />
Heroin itself has little<br />
psychoactive potency and can<br />
be regarded as a pro-drug delivering<br />
6-AM and morphine<br />
to the brain.<br />
Morphine is principally excreted<br />
directly and as the gluc-<br />
Poppy<br />
seeds<br />
Parent drug and/or<br />
metabolite<br />
Figure 17-1. Principal opiate metabolic pathways.<br />
Figure courtesy <strong>of</strong> Dr. Tai Kwong.<br />
<strong>Opioids</strong> 1: <strong>Opiates</strong> | 17<br />
uronide but, in high dose or chronic abuse situations,<br />
may be converted to hydromorphone and normorphine<br />
to a minor degree.<br />
Codeine is principally demethylated by the cytochrome<br />
P450 2D6 (CYP2D6) to morphine and norcodeine,<br />
and to a minor degree converted to hydrocodone.<br />
All four compounds are excreted as both free<br />
drugs and conjugates. Metabolism <strong>of</strong> codeine is subject<br />
to significant genetic variation <strong>of</strong> CYP2D6 polymorphisms<br />
among poor and rapid metabolizers, the incidence<br />
<strong>of</strong> which varies between ethnic groups, with, for<br />
example, poor metabolizers representing an estimated<br />
7% <strong>of</strong> Caucasians but up to 50% <strong>of</strong> Chinese. 15<br />
Hydrocodone is principally metabolized to hydromorphone,<br />
norhydrocodone, and dihydrocodeine, and<br />
to a minor degree to 6-hydromorphol.<br />
Hydromorphone is principally excreted as the conjugate<br />
and minorly as 6-hydromorphol.<br />
Oxycodone is principally converted to noroxycodone,<br />
oxymorphone, and noroxymorphone, which are<br />
excreted free and as conjugates. Minor metabolites are<br />
free and conjugated a- and b-oxycodol, noroxycodol,<br />
and oxymorphol.<br />
Oxymorphone is principally excreted as the conjugate<br />
and to a lesser degree as free and conjugated<br />
6-oxymorphol.<br />
These relationships are summarized in Figure 17-1.<br />
Excretion<br />
The opiates and their metabolites are primarily excreted<br />
in the urine and feces, and glucuronated opi-<br />
Heroin<br />
6-AM Oxymorphone<br />
Oxycodone<br />
Morphine<br />
Hydromorphone<br />
Major<br />
pathway<br />
Codeine<br />
Hydrocodone<br />
Dihydrocodeine<br />
Minor<br />
pathway<br />
Manufacturing<br />
pathway<br />
145
III A | The Toxicology Laboratory’s Test Menu: Abused Substances<br />
Table 17-3. Opioid Metabolites and Types <strong>of</strong> Specimens in Which They Can Be Detected<br />
Drug Metabolites Present in *<br />
ates/metabolites are subject to enterohepatic recirculation.<br />
Plasma clearance <strong>of</strong> codeine is 10.0 to 15.0 mL/<br />
min/kg, while that <strong>of</strong> morphine (and heroin) is 15.0 to<br />
20.0 mL/min/kg (dose-dependent).<br />
Analysis 1<br />
Specimen Types, Requirements,<br />
and Characteristics 1<br />
Specimen types, requirements, and characteristics are<br />
summarized in Table 17-3.<br />
Modes <strong>of</strong> Analysis 1<br />
The opiates are generally detected by immunoassay<br />
and/or chromatographic procedures, with confirmation<br />
by a mass spectrometric method. To some extent,<br />
the analysis <strong>of</strong> opioids is dependent on the specimen<br />
to be tested, the drug to be detected, and the circumstances<br />
surrounding the analysis. There are a number <strong>of</strong><br />
commercial immunoassays available, including enzyme<br />
immunoassays, fluorescence immunoassays, and microparticle<br />
immunoassays. Some <strong>of</strong> these are available<br />
as point-<strong>of</strong>-collection tests as well. This latter group use<br />
morphine as the calibrator, and in addition to detecting<br />
morphine, they will cross-react to morphine-6-glucuronide,<br />
codeine, hydrocodone, hydromorphone, and<br />
146<br />
Blood Urine Hair Oral Fluids<br />
Morphine Morphine X X X X<br />
Morphine-6-glucuronide X X X ?<br />
Normorphine X X<br />
Hydromorphone X<br />
Codeine Codeine X X X X<br />
Norcodeine X X X X<br />
Morphine X X X X<br />
Morphine-6-glucuronide X X X<br />
Hydrocodone X<br />
Heroin Heroin X X X<br />
6-Monoacetylmorphine X X X X<br />
Morphine X X X X<br />
Morphine-6-glucuronide X X X ?<br />
* These are dependent upon dose, duration <strong>of</strong> use, analytical sensitivity, etc.<br />
other opioids <strong>of</strong> similar chemical structure. As with all<br />
point-<strong>of</strong>-collection tests, any positive result should be<br />
confirmed by mass spectrometry.<br />
Laboratory-based immunoassays are used to detect<br />
opioids in urine, blood (or plasma), oral fluid,<br />
and hair. Commercial kits are available for morphine<br />
and opioids related in chemical structure: methadone,<br />
propoxyphene, buprenorphine, oxycodone, and fentanyl.<br />
Enzyme immunoassays are used for urine, and<br />
enzyme-linked immunosorbent assays (ELISAs) are<br />
used for other specimens. Fluorescence and microparticle<br />
assays are recommended for urine testing only.<br />
Confirmation procedures are usually based on mass<br />
spectrometry and include gas chromatography/mass<br />
spectrometry (GC-MS), gas chromatography/tandem<br />
mass spectrometry (GC-MS/MS), and the corresponding<br />
liquid chromatography (LC) techniques,<br />
LC-MS and LC-MS/MS.<br />
There are also numerous reports <strong>of</strong> detection <strong>of</strong> opioids<br />
in hair and oral fluid. Table 17-3 lists some <strong>of</strong> the<br />
major metabolites <strong>of</strong> the most commonly used opioids<br />
and the specimens in which they can be detected. For<br />
some, data may not be available for their detection in<br />
hair or oral fluid; however, it would be expected that<br />
they would be found in these specimens. Examples<br />
would be meperidine and its metabolite, normeperi-
dine; as weak bases, they should both be found in these<br />
specimens. Another factor to consider is the dose <strong>of</strong><br />
the opioid. Detection in hair is dependent on the dose,<br />
and a single dose may lead to insufficient drug being<br />
deposited in the hair shaft for detection.<br />
Known Analytical Issues and Problems 1<br />
There are a large number <strong>of</strong> opioids that will not crossreact<br />
with any <strong>of</strong> these kits and will therefore require<br />
the use <strong>of</strong> chromatographic procedures. Because the<br />
concentrations are <strong>of</strong>ten low, mass spectrometry may<br />
be necessary for their detection. Examples include naltrexone,<br />
meperidine, pentazocine, alfentanil, and tramadol.<br />
Clinical Issues<br />
Clinical Interpretation<br />
<strong>of</strong> Analytical Findings 1<br />
As with a number <strong>of</strong> drugs, the interpretation <strong>of</strong> opioid<br />
concentrations in blood and other specimens should be<br />
approached with caution. There are a number <strong>of</strong> factors<br />
that need to be considered, including the development<br />
<strong>of</strong> tolerance, the pharmacodynamics in seriously ill and<br />
geriatric patients, and whether the patient is a fast or<br />
slow metabolizer (for example, methadone). In addition,<br />
one might expect postmortem distribution with a<br />
number <strong>of</strong> these drugs.<br />
Since the introduction <strong>of</strong> workplace drug testing, the<br />
interpretation <strong>of</strong> positive urine drug tests for morphine<br />
and codeine has caused concern for medical review <strong>of</strong>ficers<br />
(MROs). Testing for morphine and codeine is<br />
routine in these programs. Morphine is a metabolite<br />
<strong>of</strong> codeine and heroin, as well as being a constituent <strong>of</strong><br />
poppy seeds. A morphine-positive result could result<br />
from the use <strong>of</strong> heroin, codeine, or morphine, or the<br />
ingestion <strong>of</strong> poppy seeds. Obviously if 6-monoacetylmorphine<br />
(6-AM) is detected with morphine, the donor<br />
has used heroin. Generally the confirmation cut<strong>of</strong>f<br />
used for both codeine and morphine is 2000 ng/mL<br />
(which reflects total codeine or total morphine), and<br />
10 ng/mL for 6-AM. Over the years, certain guidelines<br />
have been used for the interpretation <strong>of</strong> these results,<br />
as follows:<br />
After codeine use, the codeine concentration is<br />
greater than that <strong>of</strong> morphine. If the program uses a<br />
300-ng/mL cut<strong>of</strong>f, then late in the urinary excretion<br />
curve the morphine concentration may exceed that <strong>of</strong><br />
codeine.<br />
After poppy seed ingestion, there is normally very<br />
small amounts <strong>of</strong> codeine present, which <strong>of</strong>ten does<br />
not exceed the cut<strong>of</strong>f and would not be reported to the<br />
<strong>Opioids</strong> 1: <strong>Opiates</strong> | 17<br />
MRO. There are a number <strong>of</strong> reports <strong>of</strong> urine morphine<br />
concentrations in the 100s <strong>of</strong> ng/mL after the<br />
ingestion <strong>of</strong> poppy seeds, and some where concentrations<br />
<strong>of</strong> 1000s <strong>of</strong> ng/mL were reported after repeated<br />
ingestion. In ethnic communities that cook with poppy<br />
seed paste, there are reports <strong>of</strong> much higher urine morphine<br />
concentrations.<br />
In regulated drug testing programs, the MRO and<br />
the laboratory use the following procedure:<br />
6-AM is screened for against a 10-ng/mL cut<strong>of</strong>f. In<br />
6-AM confirmed positives:<br />
v If the morphine is at or above 2000 ng/mL, proceed<br />
with MRO review.<br />
v If the morphine is less than 2000 ng/mL, the<br />
MRO must first confer with the laboratory to<br />
determine if morphine was confirmed below<br />
2000 ng/mL.<br />
v If morphine was not confirmed, the MRO and<br />
the laboratory must determine if further testing is<br />
necessary.<br />
v If the laboratory finds no detectable morphine at<br />
their assay’s limit <strong>of</strong> detection, they must report<br />
this to the program.<br />
Treatment and Rehabilitation 11,16<br />
Opiate overdose classically manifests as pinpoint pupils<br />
accompanied by respiratory and CNS depression<br />
and loss <strong>of</strong> consciousness from which patients awaken<br />
after naloxone injection. Needle track-marks and other<br />
signs <strong>of</strong> intravenous drug abuse may be evident.<br />
Specific drug concentrations are usually not utilized<br />
in the acute overdose situation, but qualitative urine<br />
screening may confirm recent opiate use, although<br />
synthetic opioids are <strong>of</strong>ten not detected by these tests.<br />
Laboratory tests that are <strong>of</strong> use include electrolytes,<br />
glucose, arterial blood gases or oximetry, chest x-ray,<br />
and, to cover for the possibility <strong>of</strong> combined ingestions,<br />
stat serum acetaminophen or salicylate levels.<br />
In the emergency situation, airway management<br />
and assisted ventilation may be necessary, along with<br />
supplemental oxygen. Noncardiogenic pulmonary<br />
edema and hypotension may require emergent treatment,<br />
as would seizures or coma. Activated charcoal,<br />
if instituted rapidly, need not be accompanied by gastric<br />
emptying, and attempting to enhance elimination<br />
is obviated by the opiates’ relatively large volumes <strong>of</strong><br />
distribution and the availability <strong>of</strong> an effective antidote.<br />
Whole bowel irrigation may be appropriate for body<br />
packers and stuffers, and surgical removal may even be<br />
necessary in instances <strong>of</strong> intestinal perforation or obstruction<br />
in such cases.<br />
147
III A | The Toxicology Laboratory’s Test Menu: Abused Substances<br />
As an antidote, naloxone, an opioid antagonist without<br />
any agonist properties, may usually be given safely<br />
in doses up to 10 to 20 mg, but with the relatively short<br />
1- to 2-hour duration <strong>of</strong> naloxone effects, it is generally<br />
recommended that, after awakening, opiate coma patients<br />
should be held and/or admitted for observation<br />
for at least 6 to 12 hours. 17 Nalmefene is a newer opioid<br />
antagonist with a somewhat longer duration <strong>of</strong> effect.<br />
Complications <strong>of</strong> opiate overdose may include aspiration,<br />
rhabdomyolysis, anoxic brain injury, as well as<br />
adulterant and coingestant effects.<br />
References<br />
1. Peat MA. The <strong>Opiates</strong> [educational module]. Northfield,<br />
IL: <strong>College</strong> <strong>of</strong> <strong>American</strong> <strong>Pathologists</strong>; 2006.<br />
2. Wills S. Drugs <strong>of</strong> Abuse. 2nd ed. London, UK: Pharmaceutical<br />
Press; 2005:35-42.<br />
3. M<strong>of</strong>fat AC, Osselton MD, Widdop B, eds. Clarke’s<br />
Analysis <strong>of</strong> Drugs and Poisons. 3rd ed. London, UK: Pharmaceutical<br />
Press; 2004:845-847, 894-896, 1110-1111,<br />
1113-1114, 1302-1305, 1380-1382, 1383-1384.<br />
4. Wu AR. Personal communication, 2010 [on measured<br />
molecular masses].<br />
5. Baselt RC. Disposition <strong>of</strong> Toxic Drugs and Chemicals in<br />
Man. 8th ed. Foster City, CA: Biomedical Publications;<br />
2008:730.<br />
6. Martin WR, Sloan JW. Neuropharmacology and neurochemistry<br />
<strong>of</strong> subjective effects, analgesia, tolerance and<br />
dependence produced by narcotic analgesics. In: Martin<br />
WR, ed. Handbook <strong>of</strong> Experimental Pharmacology. Vol 45/1,<br />
Drug Addiction I: Morphine Sedative/Hypnotic and Alcohol<br />
Dependence. Berlin: Springer-Verlag; 1977:43-158.<br />
148<br />
7. Wills S. Drugs <strong>of</strong> Abuse. 2nd ed. London, UK: Pharmaceutical<br />
Press; 2005:38.<br />
8. Shults TF. Medical Review Officer Handbook. 9th ed. Research<br />
Triangle Park, NC: Quadrangle Research, LLC;<br />
2009;259.<br />
9. Rothman R B. A review <strong>of</strong> the role <strong>of</strong> anti-opioid peptides<br />
in morphine tolerance and dependence. Synapse.<br />
1992;12:129-138.<br />
10. Baselt RC. Disposition <strong>of</strong> Toxic Drugs and Chemicals in<br />
Man. 8th ed. Foster City, CA: Biomedical Publications;<br />
2008:355-360, 730-735, 745-747, 750-752, 1057-1061,<br />
1166-1172.<br />
11. Olson KR, ed. Poisoning and Drug Overdose. 4th ed. New<br />
York, NY: McGraw-Hill; 2004;286-291.<br />
12. Ling LJ, Clark RF, Erickson TB, Trestrail JH. Toxicology<br />
Secrets. Philadelphia, PA: Hanley and Belfus; 2001:105-<br />
108.<br />
13. Wills S. Drugs <strong>of</strong> Abuse. 2nd ed. London, UK: Pharmaceutical<br />
Press; 2005:55-59.<br />
14. Friedman JM, Polifka JE. The Effects <strong>of</strong> Drugs on the Fetus<br />
and Nursing Infant. Baltimore, MD: Johns Hopkins<br />
University Press; 1996:176-179, 297-301, 417-421, 454-<br />
455.<br />
15. Stout PR. <strong>Opioids</strong>. In: Ropero-Miller JD, Goldberger<br />
BA, eds. Handbook <strong>of</strong> Workplace Drug Testing. 2nd ed.<br />
Washington DC: AACC Press; 2009:289-316.<br />
16. Linden CH, Rippe JM, Irwin RS. Manual <strong>of</strong> Overdoses<br />
and Poisonings. Philadelphia, PA: Lippincott, Williams<br />
and Wilkins; 2006:161-164.<br />
17. Goldfrank K, Weisman RS, Errick JK, et al. A dosing<br />
nomogram for continuous infusion intravenous naloxone.<br />
Ann Emerg Med. 1986;15:566-570.