Isothermal process on p-V, T-V, and p-T diagrams
Isothermal process on p-V, T-V, and p-T diagrams
Isothermal process on p-V, T-V, and p-T diagrams
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
γ<br />
pV = c<strong>on</strong>stant<br />
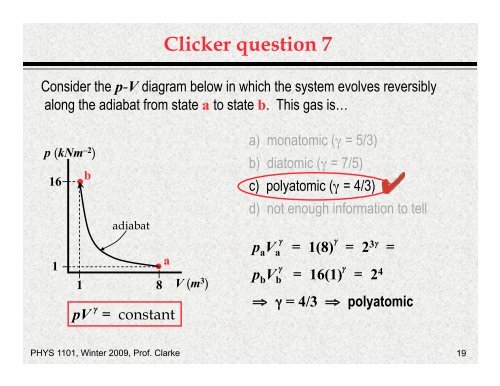
Clicker questi<strong>on</strong> 7<br />
C<strong>on</strong>sider the p-V diagram below in which the system evolves reversibly<br />
al<strong>on</strong>g the adiabat from state a to state b. This gas is…<br />
p (kNm –2 )<br />
16<br />
1<br />
b<br />
adiabat<br />
1 8<br />
a<br />
V (m 3 )<br />
a) m<strong>on</strong>atomic (γ = 5/3)<br />
b) diatomic (γ = 7/5)<br />
c) polyatomic (γ = 4/3)<br />
d) not enough informati<strong>on</strong> to tell<br />
γ γ<br />
paVa = 1(8) = 23 γ =<br />
p b V b = 16(1) = 2 4<br />
⇒ γ = 4/3 ⇒ polyatomic<br />
PHYS 1101, Winter 2009, Prof. Clarke 19<br />
γ<br />
γ