Isothermal process on p-V, T-V, and p-T diagrams
Isothermal process on p-V, T-V, and p-T diagrams
Isothermal process on p-V, T-V, and p-T diagrams
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
p<br />
p 1<br />
p 2<br />
<str<strong>on</strong>g>Isothermal</str<strong>on</strong>g> <str<strong>on</strong>g>process</str<strong>on</strong>g> <strong>on</strong> p-V, T-V, <strong>and</strong> p-T <strong>diagrams</strong><br />
V 1<br />
a<br />
T 0<br />
W<br />
Q<br />
V 2<br />
b<br />
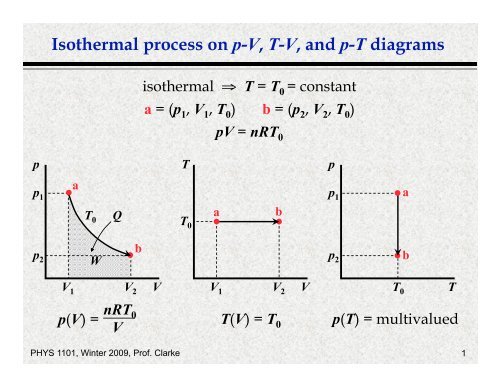
isothermal ⇒ T = T 0 = c<strong>on</strong>stant<br />
a = (p 1 , V 1 , T 0 ) b = (p 2 , V 2 , T 0 )<br />
V<br />
T<br />
T 0<br />
pV = nRT 0<br />
a<br />
V 1<br />
nRT0 p(V) = T(V) = T<br />
V<br />
0 p(T) = multivalued<br />
PHYS 1101, Winter 2009, Prof. Clarke 1<br />
b<br />
V 2<br />
V<br />
p<br />
p 1<br />
p 2<br />
T 0<br />
a<br />
b<br />
T
p<br />
p 1<br />
p 2<br />
Isochoric <str<strong>on</strong>g>process</str<strong>on</strong>g> <strong>on</strong> p-V, T-V, <strong>and</strong> p-T <strong>diagrams</strong><br />
V 0<br />
a<br />
b<br />
isochoric ⇒ V = V 0 = c<strong>on</strong>stant<br />
a = (p 1 , V 0 , T 1 ) b = (p 2 , V 0 , T 2 )<br />
V<br />
pV 0 = nRT<br />
p(V) = multivalued T(V) = multivalued<br />
T<br />
T 1<br />
V 0<br />
nRT<br />
p(T) =<br />
V0<br />
PHYS 1101, Winter 2009, Prof. Clarke 2<br />
a<br />
T2 b<br />
p2 b<br />
V<br />
p<br />
p 1<br />
T 2<br />
T 1<br />
a<br />
T
p<br />
p 0<br />
Isobaric <str<strong>on</strong>g>process</str<strong>on</strong>g> <strong>on</strong> p-V, T-V, <strong>and</strong> p-T <strong>diagrams</strong><br />
a<br />
V 1<br />
Q<br />
W<br />
p(V) = p 0<br />
b<br />
V 2<br />
isobaric ⇒ p = p 0 = c<strong>on</strong>stant<br />
a = (p 0 , V 1 , T 1 ) b = (p 0 , V 2 , T 2 )<br />
V<br />
T<br />
T 2<br />
T 1<br />
a<br />
p 0 V = nRT<br />
V 1<br />
p0V T(V) =<br />
nR<br />
p(T) = p 0<br />
PHYS 1101, Winter 2009, Prof. Clarke 3<br />
b<br />
V 2<br />
V<br />
p<br />
p 0<br />
a<br />
T 1<br />
b<br />
T 2<br />
T
p (Nm –2 )<br />
10 5<br />
a<br />
1<br />
T 0<br />
V c<br />
Clicker questi<strong>on</strong> 1<br />
C<strong>on</strong>sider the p-V diagram below in which the system evolves from a → b<br />
→ c. If T0 ~ 240K (<strong>and</strong> thus RT0 = 2,000 J mol –1 ), how many moles of<br />
gas, n, are in the system?<br />
a) 5<br />
p c<br />
isobar<br />
b<br />
isotherm<br />
c<br />
isochor<br />
V (m 3 )<br />
b) 10 5<br />
c) 50<br />
d) 1,000<br />
e) Not enough informati<strong>on</strong> to tell<br />
first law of thermodynamics: ΔE int = Q – W ( = nC V ΔT )<br />
ideal gas law: pV = nRT<br />
PHYS 1101, Winter 2009, Prof. Clarke 4
p (Nm –2 )<br />
10 5<br />
p c<br />
a<br />
1<br />
T 0<br />
V c<br />
Clicker questi<strong>on</strong> 1<br />
C<strong>on</strong>sider the p-V diagram below in which the system evolves from a → b<br />
→ c. If T0 ~ 240K (<strong>and</strong> thus RT0 = 2,000 J mol –1 ), how many moles of<br />
gas, n, are in the system?<br />
a) 5<br />
isobar<br />
b<br />
isotherm<br />
c<br />
isochor<br />
V (m 3 )<br />
b) 10 5<br />
c) 50<br />
d) 1,000<br />
e) Not enough informati<strong>on</strong> to tell<br />
pV 100,000<br />
n = = = 50<br />
RT0 2,000<br />
first law of thermodynamics: ΔE int = Q – W ( = nC V ΔT )<br />
ideal gas law: pV = nRT<br />
PHYS 1101, Winter 2009, Prof. Clarke 5
Clicker questi<strong>on</strong> 2<br />
C<strong>on</strong>sider the p-V diagram below in which the system evolves from a → b<br />
→ c. What is V c , the volume at state c?<br />
p (Nm –2 )<br />
10 5<br />
p c<br />
a<br />
1<br />
T 0<br />
V c<br />
b<br />
c<br />
V (m 3 )<br />
a) 0.5 m 3<br />
b) 2.0 m 3<br />
c) 4.0 m 3<br />
d) 8.0 m 3<br />
e) Not enough informati<strong>on</strong> to tell<br />
first law of thermodynamics: ΔE int = Q – W ( = nC V ΔT )<br />
ideal gas law: pV = nRT<br />
PHYS 1101, Winter 2009, Prof. Clarke 6
Clicker questi<strong>on</strong> 2<br />
C<strong>on</strong>sider the p-V diagram below in which the system evolves from a → b<br />
→ c. What is V c , the volume at state c?<br />
p (Nm –2 )<br />
10 5<br />
p c<br />
a<br />
1<br />
T 0<br />
V c<br />
b<br />
c<br />
V (m 3 )<br />
a) 0.5 m 3<br />
b) 2.0 m 3<br />
c) 4.0 m 3<br />
d) 8.0 m 3<br />
e) Not enough informati<strong>on</strong> to tell<br />
need to know p c<br />
first law of thermodynamics: ΔE int = Q – W ( = nC V ΔT )<br />
ideal gas law: pV = nRT<br />
PHYS 1101, Winter 2009, Prof. Clarke 7
Clicker questi<strong>on</strong> 3<br />
C<strong>on</strong>sider the p-V diagram below in which the system evolves from a → b<br />
→ c. What is V c , the volume at state c?<br />
p (Nm –2 )<br />
10 5<br />
5 ×10 4<br />
a<br />
1<br />
T 0<br />
V c<br />
b<br />
c<br />
V (m 3 )<br />
a) 0.5 m 3<br />
b) 2.0 m 3<br />
c) 4.0 m 3<br />
d) 8.0 m 3<br />
e) Not enough informati<strong>on</strong> to tell<br />
first law of thermodynamics: ΔE int = Q – W ( = nC V ΔT )<br />
ideal gas law: pV = nRT<br />
PHYS 1101, Winter 2009, Prof. Clarke 8
Clicker questi<strong>on</strong> 3<br />
C<strong>on</strong>sider the p-V diagram below in which the system evolves from a → b<br />
→ c. What is V c , the volume at state c?<br />
p (Nm –2 )<br />
10 5<br />
5 ×10 4<br />
a<br />
1<br />
T 0<br />
V c<br />
b<br />
c<br />
V (m 3 )<br />
a) 0.5 m 3<br />
b) 2.0 m 3<br />
c) 4.0 m 3<br />
d) 8.0 m 3<br />
e) Not enough informati<strong>on</strong> to tell<br />
pcVc = paVa ⇒ Vc = Va = 2 m3 pa pc first law of thermodynamics: ΔE int = Q – W ( = nC V ΔT )<br />
ideal gas law: pV = nRT<br />
PHYS 1101, Winter 2009, Prof. Clarke 9
Clicker questi<strong>on</strong> 4<br />
C<strong>on</strong>sider the p-V diagram below in which the system evolves from a → b<br />
→ c. What is the net change in internal energy, ΔE int ?<br />
p (Nm –2 )<br />
10 5<br />
5 ×10 4<br />
a<br />
1<br />
T 0<br />
2<br />
b<br />
c<br />
V (m 3 )<br />
a) 0 J<br />
b) 5.0 ×10 4 J<br />
c) about 7.0 ×10 4 J<br />
d) 10 5 J<br />
e) Not enough informati<strong>on</strong> to tell<br />
first law of thermodynamics: ΔE int = Q – W ( = nC V ΔT )<br />
ideal gas law: pV = nRT<br />
PHYS 1101, Winter 2009, Prof. Clarke 10
Clicker questi<strong>on</strong> 4<br />
C<strong>on</strong>sider the p-V diagram below in which the system evolves from a → b<br />
→ c. What is the net change in internal energy, ΔE int ?<br />
p (Nm –2 )<br />
10 5<br />
5 ×10 4<br />
a<br />
1<br />
T 0<br />
2<br />
b<br />
c<br />
V (m 3 )<br />
a) 0 J<br />
b) 5.0 ×10 4 J<br />
c) about 7.0 ×10 4 J<br />
d) 10 5 J<br />
e) Not enough informati<strong>on</strong> to tell<br />
ΔE int = nC V ΔT<br />
first law of thermodynamics: ΔE int = Q – W ( = nC V ΔT )<br />
ideal gas law: pV = nRT<br />
PHYS 1101, Winter 2009, Prof. Clarke 11
Clicker questi<strong>on</strong> 5<br />
C<strong>on</strong>sider the p-V diagram below in which the system evolves from a → b<br />
→ c. What is the net work d<strong>on</strong>e by the system <strong>on</strong> its envir<strong>on</strong>ment, W?<br />
p (Nm –2 )<br />
10 5<br />
5 ×10 4<br />
a<br />
1<br />
T 0<br />
2<br />
b<br />
c<br />
V (m 3 )<br />
a) 0 J<br />
b) 5.0 ×10 4 J<br />
c) about 7.0 ×10 4 J<br />
d) 10 5 J<br />
e) Not enough informati<strong>on</strong> to tell<br />
first law of thermodynamics: ΔE int = Q – W ( = nC V ΔT )<br />
ideal gas law: pV = nRT<br />
PHYS 1101, Winter 2009, Prof. Clarke 12
Clicker questi<strong>on</strong> 5<br />
C<strong>on</strong>sider the p-V diagram below in which the system evolves from a → b<br />
→ c. What is the net work d<strong>on</strong>e by the system <strong>on</strong> its envir<strong>on</strong>ment, W?<br />
p (Nm –2 )<br />
10 5<br />
5 ×10 4<br />
a<br />
1<br />
T 0<br />
W<br />
2<br />
b<br />
c<br />
V (m 3 )<br />
a) 0 J<br />
b) 5.0 ×10 4 J<br />
c) about 7.0 ×10 4 J<br />
d) 10 5 J<br />
e) Not enough informati<strong>on</strong> to tell<br />
first law of thermodynamics: ΔE int = Q – W ( = nC V ΔT )<br />
ideal gas law: pV = nRT<br />
PHYS 1101, Winter 2009, Prof. Clarke 13
Clicker questi<strong>on</strong> 6<br />
C<strong>on</strong>sider the p-V diagram below in which the system evolves from a → b<br />
→ c. What is the net heat transferred into the system, Q?<br />
p (Nm –2 )<br />
10 5<br />
5 ×10 4<br />
a<br />
1<br />
T 0<br />
2<br />
b<br />
c<br />
V (m 3 )<br />
a) –5.0 ×10 4 J<br />
b) 5.0 ×10 4 J<br />
c) –10 5 J<br />
d) 10 5 J<br />
e) Not enough informati<strong>on</strong> to tell<br />
first law of thermodynamics: ΔE int = Q – W ( = nC V ΔT )<br />
ideal gas law: pV = nRT<br />
PHYS 1101, Winter 2009, Prof. Clarke 14
Clicker questi<strong>on</strong> 6<br />
C<strong>on</strong>sider the p-V diagram below in which the system evolves from a → b<br />
→ c. What is the net heat transferred into the system, Q?<br />
p (Nm –2 )<br />
10 5<br />
5 ×10 4<br />
a<br />
1<br />
T 0<br />
2<br />
b<br />
c<br />
V (m 3 )<br />
a) –5.0 ×10 4 J<br />
b) 5.0 ×10 4 J<br />
c) –10 5 J<br />
d) 10 5 J<br />
e) Not enough informati<strong>on</strong> to tell<br />
Q = ΔE int + W = 0 + 10 5 J<br />
first law of thermodynamics: ΔE int = Q – W ( = nC V ΔT )<br />
ideal gas law: pV = nRT<br />
PHYS 1101, Winter 2009, Prof. Clarke 15
type of gas degrees of<br />
freedom<br />
( f )<br />
Internal Energy (revisited)<br />
f<br />
2<br />
E int = nC V T = nRT = NkT C p = C V + R<br />
n = number of moles; 1 mole = 6.0221 × 10 22 particles (N A )<br />
N = number of particles<br />
R = gas c<strong>on</strong>stant = 8.3147 J mol –1 K –1<br />
k = Boltzmann’s c<strong>on</strong>stant = 1.3807 × 10 –23 J K –1<br />
specific heat at<br />
c<strong>on</strong>stant<br />
volume (C V )<br />
internal<br />
energy<br />
(E int )<br />
specific heat at<br />
c<strong>on</strong>stant<br />
pressure (C p )<br />
3 3<br />
5<br />
m<strong>on</strong>atomic 3 R nRT R<br />
2 2<br />
2<br />
5 5<br />
7<br />
diatomic 5 R nRT R<br />
2 2<br />
2<br />
polyatomic (≥3) ~6 3 R 3 nRT 4 R<br />
f<br />
2<br />
γ<br />
(C p /C V )<br />
PHYS 1101, Winter 2009, Prof. Clarke 16<br />
5<br />
3<br />
7<br />
5<br />
4<br />
3
p<br />
p 1<br />
p 2<br />
reversible<br />
a = (p 1 , V 1 , T 1 )<br />
b = (p 2 , V 2 , T 2 )<br />
γ<br />
pV = c<strong>on</strong>stant<br />
T 2<br />
V 1<br />
a<br />
T 1<br />
V 2<br />
Adiabatic <str<strong>on</strong>g>process</str<strong>on</strong>g>es<br />
adiabat<br />
b<br />
isotherms<br />
V<br />
isotherm<br />
PHYS 1101, Winter 2009, Prof. Clarke 17<br />
p<br />
p 1<br />
p 2<br />
V 1<br />
irreversible<br />
a = (p 1 , V 1 , T 0 )<br />
b = (p 2 , V 2 , T 0 )<br />
p 1 V 1 = p 2 V 2<br />
a<br />
T 0<br />
V 2<br />
b<br />
V
γ<br />
pV = c<strong>on</strong>stant<br />
Clicker questi<strong>on</strong> 7<br />
C<strong>on</strong>sider the p-V diagram below in which the system evolves reversibly<br />
al<strong>on</strong>g the adiabat from state a to state b. This gas is…<br />
p (kNm –2 )<br />
16<br />
1<br />
b<br />
adiabat<br />
1 8<br />
a<br />
V (m 3 )<br />
a) m<strong>on</strong>atomic (γ = 5/3)<br />
b) diatomic (γ = 7/5)<br />
c) polyatomic (γ = 4/3)<br />
d) not enough informati<strong>on</strong> to tell<br />
PHYS 1101, Winter 2009, Prof. Clarke 18
γ<br />
pV = c<strong>on</strong>stant<br />
Clicker questi<strong>on</strong> 7<br />
C<strong>on</strong>sider the p-V diagram below in which the system evolves reversibly<br />
al<strong>on</strong>g the adiabat from state a to state b. This gas is…<br />
p (kNm –2 )<br />
16<br />
1<br />
b<br />
adiabat<br />
1 8<br />
a<br />
V (m 3 )<br />
a) m<strong>on</strong>atomic (γ = 5/3)<br />
b) diatomic (γ = 7/5)<br />
c) polyatomic (γ = 4/3)<br />
d) not enough informati<strong>on</strong> to tell<br />
γ γ<br />
paVa = 1(8) = 23 γ =<br />
p b V b = 16(1) = 2 4<br />
⇒ γ = 4/3 ⇒ polyatomic<br />
PHYS 1101, Winter 2009, Prof. Clarke 19<br />
γ<br />
γ
γ<br />
pV = c<strong>on</strong>stant<br />
Clicker questi<strong>on</strong> 8<br />
C<strong>on</strong>sider the p-V diagram below in which the system evolves reversibly<br />
al<strong>on</strong>g the adiabat from state a to state b. How much heat is transferred to<br />
the system?<br />
a) 0 J<br />
p (kNm –2 )<br />
16<br />
1<br />
b<br />
adiabat<br />
1 8<br />
a<br />
V (m 3 )<br />
b) 8 kJ<br />
c) 16 kJ<br />
d) 128 kJ<br />
e) not enough informati<strong>on</strong> to tell<br />
PHYS 1101, Winter 2009, Prof. Clarke 20
γ<br />
pV = c<strong>on</strong>stant<br />
Clicker questi<strong>on</strong> 8<br />
C<strong>on</strong>sider the p-V diagram below in which the system evolves reversibly<br />
al<strong>on</strong>g the adiabat from state a to state b. How much heat, Q, is<br />
transferred to the system?<br />
a) 0 J<br />
p (kNm –2 )<br />
16<br />
1<br />
b<br />
adiabat<br />
1 8<br />
a<br />
V (m 3 )<br />
b) 8 kJ<br />
c) 16 kJ<br />
d) 128 kJ<br />
e) not enough informati<strong>on</strong> to tell<br />
By definiti<strong>on</strong>, Q = 0 for all adiabatic<br />
<str<strong>on</strong>g>process</str<strong>on</strong>g>es.<br />
PHYS 1101, Winter 2009, Prof. Clarke 21
Clicker questi<strong>on</strong> 9<br />
C<strong>on</strong>sider the p-V diagram below in which the system evolves reversibly<br />
al<strong>on</strong>g the adiabat from state a to state b. How much work, W, does the<br />
system do <strong>on</strong> its envir<strong>on</strong>ment?<br />
a) 20 kJ b) –20 kJ<br />
p (kNm –2 )<br />
16<br />
1<br />
b<br />
adiabat<br />
1 8<br />
a<br />
V (m 3 )<br />
c) 24 kJ d) –24 kJ<br />
e) 32 kJ f) –32 kJ<br />
g) not enough informati<strong>on</strong> to tell<br />
E int = nC V T = n3RT (for a polyatomic gas)<br />
first law: ΔE int = Q – W ideal gas law: pV = nRT<br />
PHYS 1101, Winter 2009, Prof. Clarke 22
Clicker questi<strong>on</strong> 9<br />
C<strong>on</strong>sider the p-V diagram below in which the system evolves reversibly<br />
al<strong>on</strong>g the adiabat from state a to state b. How much work, W, does the<br />
system do <strong>on</strong> its envir<strong>on</strong>ment?<br />
a) 20 kJ b) –20 kJ<br />
p (kNm –2 )<br />
16<br />
1<br />
b<br />
W<br />
adiabat<br />
1 8<br />
a<br />
V (m 3 )<br />
c) 24 kJ d) –24 kJ<br />
e) 32 kJ f) –32 kJ<br />
g) not enough informati<strong>on</strong> to tell<br />
ΔE int = 0 – W = 3Δ(nRT) = 3Δ(pV)<br />
= 3(16 – 8) = 24 ⇒ W = –24 kJ<br />
E int = nC V T = n3RT (for a polyatomic gas)<br />
first law: ΔE int = Q – W ideal gas law: pV = nRT<br />
PHYS 1101, Winter 2009, Prof. Clarke 23
p<br />
p 1<br />
p 2<br />
p 3<br />
p 4<br />
S 4<br />
S 3<br />
a<br />
V 1<br />
e<br />
S 2<br />
S 1<br />
Summary of Processes<br />
isentrop: ΔS = 0<br />
(reversible adiabat: Q = 0)<br />
V 2<br />
d<br />
isobar: Δp = 0<br />
isochor: ΔV = 0<br />
isotherm: ΔT = 0<br />
T 2<br />
V<br />
T 1<br />
T 3<br />
free expansi<strong>on</strong> (irreversible adiabat: Q = 0)<br />
b<br />
c<br />
a = (p 1 , V 1 , T 2 , S 3 )<br />
b = (p 1 , V 2 , T 1 , S 1 )<br />
c = (p 2 , V 2 , T 2 , S 2 )<br />
d = (p 4 , V 2 , T 3 , S 3 )<br />
e = (p 3 , V 1 , T 3 , S 4 )<br />
PHYS 1101, Winter 2009, Prof. Clarke 24
Summary of Processes<br />
<str<strong>on</strong>g>process</str<strong>on</strong>g> W Q<br />
ΔE int =<br />
nC V ΔT<br />
pCp pCV isobar p(V2 – V1 ) (V2 – V1 ) (V2 – V1 ) nCp ln ( )<br />
R<br />
V1 V2 isotherm nRT ln nRT ln 0 nR ln<br />
p1V1 – p2V2 p2V2 – p1V1 isentrop 0 0<br />
γ – 1<br />
γ – 1<br />
free<br />
expansi<strong>on</strong><br />
( V2 V1 )<br />
R<br />
isochor 0 (p2 – p1 ) (p2 – p1 ) nCV ln ( p VCV VCV 2<br />
R<br />
R<br />
p1 ( V2 V1 0 0 0 nR ln<br />
PHYS 1101, Winter 2009, Prof. Clarke 25<br />
)<br />
ΔS<br />
( V 2<br />
V 1<br />
( V 2<br />
V 1<br />
)<br />
)<br />
)
p<br />
a<br />
V<br />
All <str<strong>on</strong>g>process</str<strong>on</strong>g>es <strong>on</strong> p-V, T-V, <strong>and</strong> T-S <strong>diagrams</strong><br />
p<br />
T<br />
S<br />
V<br />
T<br />
a<br />
V<br />
An isobar (p), isotherm (T), isentrop (S), <strong>and</strong> isochor (V)<br />
emanating from the same initial state (a) as manifest <strong>on</strong><br />
a p-V, T-V, <strong>and</strong> a T-S diagram.<br />
PHYS 1101, Winter 2009, Prof. Clarke 26<br />
p<br />
T<br />
S<br />
V<br />
T<br />
V<br />
a<br />
S<br />
p<br />
T<br />
S
p<br />
b<br />
Clicker questi<strong>on</strong> 10<br />
C<strong>on</strong>sider the p-V diagram below in which n = 1 mole of gas evolves reversibly<br />
from state a to state b al<strong>on</strong>g the path shown. What is the net change in<br />
entropy? (Note, e = 2.71828 = Euler’s number, <strong>and</strong> thus ln(e) = 1.)<br />
a<br />
1 e<br />
some formulae, in case they help…<br />
V<br />
a) C p b) C p e<br />
c) C V d) C V e<br />
e) R f) R e<br />
g) no where near enough informati<strong>on</strong>!!<br />
ΔS = nC p ln (isobar)<br />
ΔS = nC V ln (isochor)<br />
ΔS = nR ln (isotherm)<br />
PHYS 1101, Winter 2009, Prof. Clarke 27<br />
)<br />
( V2 V1 ( )<br />
p2 p1 ( )<br />
V2 V1
Clicker questi<strong>on</strong> 10<br />
C<strong>on</strong>sider the p-V diagram below in which n = 1 mole of gas evolves revers<br />
-ibly from state a to state b al<strong>on</strong>g the path shown. What is the net change in<br />
entropy? (Note, e = 2.71828 = Euler’s number, <strong>and</strong> thus ln(e) = 1.)<br />
p<br />
a<br />
1 e<br />
Since S is a state variable, it doesn’t<br />
matter which path from a to b you<br />
choose. Thus, choose the isobar.<br />
b<br />
V<br />
a) C p b) C p e<br />
c) C V d) C V e<br />
e) R f) R e<br />
g) Yes there is!!<br />
ΔS = nC p ln (isobar)<br />
ΔS = nC V ln (isochor)<br />
ΔS = nR ln (isotherm)<br />
PHYS 1101, Winter 2009, Prof. Clarke 28<br />
)<br />
( V2 V1 ( )<br />
p2 p1 ( )<br />
V2 V1
Clicker questi<strong>on</strong> 11<br />
The evoluti<strong>on</strong> of a system from state a to state b is shown <strong>on</strong> both the p-V<br />
<strong>and</strong> T-S <strong>diagrams</strong> below. What is the change in internal energy?<br />
p (Nm –2 )<br />
2,000<br />
b<br />
a<br />
1 V (m3 2 )<br />
T (K)<br />
500<br />
250<br />
b<br />
10 S (JK –1 30 )<br />
a) 2000 J b) –2000 J c) 5000 J<br />
d) –5000 J e) 7000 J f) –7000 J<br />
PHYS 1101, Winter 2009, Prof. Clarke 29<br />
a<br />
ΔE int = Q – W<br />
= nC V ΔT<br />
pV = nRT
Clicker questi<strong>on</strong> 11<br />
The evoluti<strong>on</strong> of a system from state a to state b is shown <strong>on</strong> both the p-V<br />
<strong>and</strong> T-S <strong>diagrams</strong> below. What is the change in internal energy?<br />
p (Nm –2 )<br />
2,000<br />
b<br />
a<br />
W = –2000 J<br />
1 V (m3 2 )<br />
T (K)<br />
500<br />
250<br />
b<br />
Q –7500 J<br />
><br />
~<br />
10 S (JK –1 30 )<br />
Q ~ –7000 J<br />
W = –2000 J<br />
ΔE int = Q – W<br />
a) 2000 J b) –2000 J c) 5000 J<br />
d) –5000 J e) 7000 J f) –7000 J<br />
~ –5000 J<br />
PHYS 1101, Winter 2009, Prof. Clarke 30<br />
a
Clicker questi<strong>on</strong> 12<br />
The evoluti<strong>on</strong> of a system from state a to state b is shown <strong>on</strong> both the p-V<br />
<strong>and</strong> T-S <strong>diagrams</strong> below. About how many moles of gas are in the<br />
system? (Take R = 8.)<br />
p (Nm –2 )<br />
2,000<br />
b<br />
a<br />
1 V (m3 2 )<br />
T (K)<br />
500<br />
250<br />
a) 0.5 b) 1 c) 1.5<br />
d) 2 e) not enough informati<strong>on</strong> to tell<br />
b<br />
10 S (JK –1 30 )<br />
PHYS 1101, Winter 2009, Prof. Clarke 31<br />
a<br />
ΔE int = Q – W<br />
= nC V ΔT<br />
pV = nRT
Clicker questi<strong>on</strong> 12<br />
The evoluti<strong>on</strong> of a system from state a to state b is shown <strong>on</strong> both the p-V<br />
<strong>and</strong> T-S <strong>diagrams</strong> below. About how many moles of gas are in the<br />
system? (Take R = 8.)<br />
p (Nm –2 )<br />
2,000<br />
b<br />
a<br />
1 V (m3 2 )<br />
T (K)<br />
500<br />
250<br />
a) 0.5 b) 1 c) 1.5<br />
d) 2 e) not enough informati<strong>on</strong> to tell<br />
b<br />
10 S (JK –1 30 )<br />
PHYS 1101, Winter 2009, Prof. Clarke 32<br />
a<br />
pV = nRT<br />
at state b:<br />
pV = 2000<br />
RT ~ 2000<br />
⇒ n ~ 1
Clicker questi<strong>on</strong> 13<br />
The evoluti<strong>on</strong> of a system from state a to state b is shown <strong>on</strong> both the p-V<br />
<strong>and</strong> T-S <strong>diagrams</strong> below. With n = 1 <strong>and</strong> ΔE int = –5000 J, this gas is:<br />
p (Nm –2 )<br />
2,000<br />
b<br />
a<br />
1 V (m3 2 )<br />
T (K)<br />
500<br />
250<br />
a) m<strong>on</strong>atomic b) diatomic c) polyatomic<br />
d) not enough informati<strong>on</strong> to tell<br />
b<br />
10 S (JK –1 30 )<br />
PHYS 1101, Winter 2009, Prof. Clarke 33<br />
a<br />
ΔE int = nC V ΔT<br />
C V ~ 12 (m<strong>on</strong>atomic)<br />
C V ~ 21 (diatomic)<br />
C V ~ 25 (polyatomic)
Clicker questi<strong>on</strong> 13<br />
The evoluti<strong>on</strong> of a system from state a to state b is shown <strong>on</strong> both the p-V<br />
<strong>and</strong> T-S <strong>diagrams</strong> below. With n = 1 <strong>and</strong> ΔE int = –5000 J, this gas is:<br />
p (Nm –2 )<br />
2,000<br />
b<br />
a<br />
1 V (m3 2 )<br />
T (K)<br />
500<br />
250<br />
a) m<strong>on</strong>atomic b) diatomic c) polyatomic<br />
d) not enough informati<strong>on</strong> to tell<br />
b<br />
10 S (JK –1 30 )<br />
PHYS 1101, Winter 2009, Prof. Clarke 34<br />
a<br />
ΔE int = nC V ΔT<br />
C V ~ 12 (m<strong>on</strong>atomic)<br />
C V ~ 21 (diatomic)<br />
C V ~ 25 (polyatomic)
p<br />
a<br />
Three pictorial representati<strong>on</strong>s of an engine<br />
Q out<br />
W > 0<br />
Q in<br />
In a complete thermodynamical<br />
cycle, gas<br />
exp<strong>and</strong>s at high pressure<br />
<strong>and</strong> compresses at low<br />
pressure allowing work,<br />
W, to be extracted in<br />
each cycle.<br />
c<br />
V<br />
Q in<br />
a→c<br />
T H<br />
expansi<strong>on</strong><br />
stroke<br />
c→a<br />
T C<br />
compressi<strong>on</strong><br />
stroke<br />
PHYS 1101, Winter 2009, Prof. Clarke 35<br />
Q out<br />
Q H = Q in<br />
Q C = Q out<br />
W out = W
T<br />
T H<br />
T C<br />
a<br />
S 1<br />
Maximum efficiency <strong>and</strong> the Carnot cycle<br />
Q in<br />
Q out<br />
c<br />
S 2<br />
S<br />
T<br />
T H<br />
T C<br />
a<br />
d<br />
S 1<br />
Q in<br />
W > 0 W > 0<br />
n<strong>on</strong>-optimal thermo<br />
-dynamical cycle for<br />
an engine<br />
Q out<br />
PHYS 1101, Winter 2009, Prof. Clarke 36<br />
b<br />
c<br />
S 2<br />
S<br />
optimal thermodyn<br />
-amical cycle for an<br />
engine (Carnot cycle)<br />
p<br />
a<br />
S 1<br />
T C<br />
Q in<br />
S 2<br />
d<br />
adiabats<br />
b<br />
isotherms<br />
T H<br />
c<br />
Qout V<br />
the Carnot engine cycle<br />
<strong>on</strong> a p-V diagram<br />
Note that for an engine, the thermodynamical cycle is always clockwise.
p<br />
a<br />
Q in<br />
W < 0<br />
Q out<br />
For a refrigerator, the<br />
cycle is always<br />
counterclockwise.<br />
Expansi<strong>on</strong> happens at<br />
low pressure,<br />
compressi<strong>on</strong> at high<br />
pressure <strong>and</strong> this takes<br />
work. Heat is drawn in at<br />
T C <strong>and</strong> expelled at T H .<br />
c<br />
V<br />
Refrigerators (heat pumps)<br />
Q C = Q in<br />
Q H = Q out<br />
W in = –W<br />
PHYS 1101, Winter 2009, Prof. Clarke 37<br />
p<br />
a<br />
S 1<br />
T C<br />
S2 Qout d<br />
adiabats<br />
b<br />
isotherms<br />
T H<br />
Q in<br />
c<br />
V<br />
As for an engine, the most<br />
optimal thermodynamical<br />
cycle for a refrigerator is the<br />
Carnot cycle traversed in<br />
the counterclockwise<br />
directi<strong>on</strong> (opposite to the<br />
engine).