AN SN1 REACTION. t-AMYL CHLORIDE FROM THE ...
AN SN1 REACTION. t-AMYL CHLORIDE FROM THE ... AN SN1 REACTION. t-AMYL CHLORIDE FROM THE ...
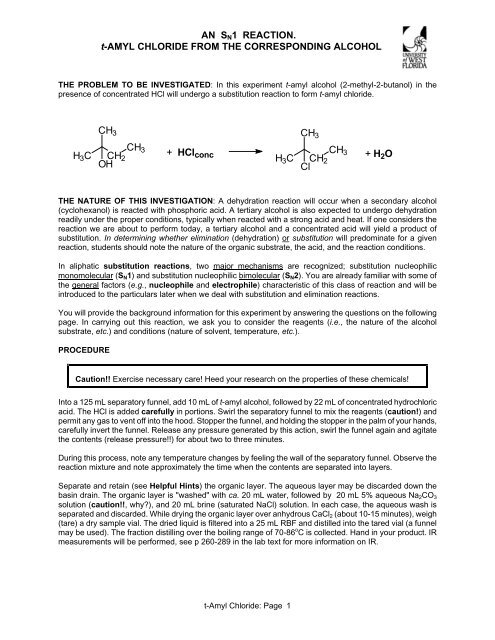
AN SN1 REACTION. t-AMYL CHLORIDE FROM THE CORRESPONDING ALCOHOL THE PROBLEM TO BE INVESTIGATED: In this experiment t-amyl alcohol (2-methyl-2-butanol) in the presence of concentrated HCl will undergo a substitution reaction to form t-amyl chloride. CH3 CH3 H3C CH2 OH THE NATURE OF THIS INVESTIGATION: A dehydration reaction will occur when a secondary alcohol (cyclohexanol) is reacted with phosphoric acid. A tertiary alcohol is also expected to undergo dehydration readily under the proper conditions, typically when reacted with a strong acid and heat. If one considers the reaction we are about to perform today, a tertiary alcohol and a concentrated acid will yield a product of substitution. In determining whether elimination (dehydration) or substitution will predominate for a given reaction, students should note the nature of the organic substrate, the acid, and the reaction conditions. In aliphatic substitution reactions, two major mechanisms are recognized; substitution nucleophilic monomolecular (SN1) and substitution nucleophilic bimolecular (SN2). You are already familiar with some of the general factors (e.g., nucleophile and electrophile) characteristic of this class of reaction and will be introduced to the particulars later when we deal with substitution and elimination reactions. You will provide the background information for this experiment by answering the questions on the following page. In carrying out this reaction, we ask you to consider the reagents (i.e., the nature of the alcohol substrate, etc.) and conditions (nature of solvent, temperature, etc.). PROCEDURE + HClconc Caution!! Exercise necessary care! Heed your research on the properties of these chemicals! Into a 125 mL separatory funnel, add 10 mL of t-amyl alcohol, followed by 22 mL of concentrated hydrochloric acid. The HCl is added carefully in portions. Swirl the separatory funnel to mix the reagents (caution!) and permit any gas to vent off into the hood. Stopper the funnel, and holding the stopper in the palm of your hands, carefully invert the funnel. Release any pressure generated by this action, swirl the funnel again and agitate the contents (release pressure!!) for about two to three minutes. During this process, note any temperature changes by feeling the wall of the separatory funnel. Observe the reaction mixture and note approximately the time when the contents are separated into layers. Separate and retain (see Helpful Hints) the organic layer. The aqueous layer may be discarded down the basin drain. The organic layer is "washed" with ca. 20 mL water, followed by 20 mL 5% aqueous Na2CO3 solution (caution!!, why?), and 20 mL brine (saturated NaCl) solution. In each case, the aqueous wash is separated and discarded. While drying the organic layer over anhydrous CaCl2 (about 10-15 minutes), weigh (tare) a dry sample vial. The dried liquid is filtered into a 25 mL RBF and distilled into the tared vial (a funnel may be used). The fraction distilling over the boiling range of 70-86 o C is collected. Hand in your product. IR measurements will be performed, see p 260-289 in the lab text for more information on IR. H3C t-Amyl Chloride: Page 1 CH3 CH2 Cl CH 3 + H2O
<strong>AN</strong> <strong>SN1</strong> <strong>REACTION</strong>.<br />
t-<strong>AMYL</strong> <strong>CHLORIDE</strong> <strong>FROM</strong> <strong>THE</strong> CORRESPONDING ALCOHOL<br />
<strong>THE</strong> PROBLEM TO BE INVESTIGATED: In this experiment t-amyl alcohol (2-methyl-2-butanol) in the<br />
presence of concentrated HCl will undergo a substitution reaction to form t-amyl chloride.<br />
CH3<br />
CH3<br />
H3C CH2<br />
OH<br />
<strong>THE</strong> NATURE OF THIS INVESTIGATION: A dehydration reaction will occur when a secondary alcohol<br />
(cyclohexanol) is reacted with phosphoric acid. A tertiary alcohol is also expected to undergo dehydration<br />
readily under the proper conditions, typically when reacted with a strong acid and heat. If one considers the<br />
reaction we are about to perform today, a tertiary alcohol and a concentrated acid will yield a product of<br />
substitution. In determining whether elimination (dehydration) or substitution will predominate for a given<br />
reaction, students should note the nature of the organic substrate, the acid, and the reaction conditions.<br />
In aliphatic substitution reactions, two major mechanisms are recognized; substitution nucleophilic<br />
monomolecular (<strong>SN1</strong>) and substitution nucleophilic bimolecular (SN2). You are already familiar with some of<br />
the general factors (e.g., nucleophile and electrophile) characteristic of this class of reaction and will be<br />
introduced to the particulars later when we deal with substitution and elimination reactions.<br />
You will provide the background information for this experiment by answering the questions on the following<br />
page. In carrying out this reaction, we ask you to consider the reagents (i.e., the nature of the alcohol<br />
substrate, etc.) and conditions (nature of solvent, temperature, etc.).<br />
PROCEDURE<br />
+ HClconc<br />
Caution!! Exercise necessary care! Heed your research on the properties of these chemicals!<br />
Into a 125 mL separatory funnel, add 10 mL of t-amyl alcohol, followed by 22 mL of concentrated hydrochloric<br />
acid. The HCl is added carefully in portions. Swirl the separatory funnel to mix the reagents (caution!) and<br />
permit any gas to vent off into the hood. Stopper the funnel, and holding the stopper in the palm of your hands,<br />
carefully invert the funnel. Release any pressure generated by this action, swirl the funnel again and agitate<br />
the contents (release pressure!!) for about two to three minutes.<br />
During this process, note any temperature changes by feeling the wall of the separatory funnel. Observe the<br />
reaction mixture and note approximately the time when the contents are separated into layers.<br />
Separate and retain (see Helpful Hints) the organic layer. The aqueous layer may be discarded down the<br />
basin drain. The organic layer is "washed" with ca. 20 mL water, followed by 20 mL 5% aqueous Na2CO3<br />
solution (caution!!, why?), and 20 mL brine (saturated NaCl) solution. In each case, the aqueous wash is<br />
separated and discarded. While drying the organic layer over anhydrous CaCl2 (about 10-15 minutes), weigh<br />
(tare) a dry sample vial. The dried liquid is filtered into a 25 mL RBF and distilled into the tared vial (a funnel<br />
may be used). The fraction distilling over the boiling range of 70-86 o C is collected. Hand in your product. IR<br />
measurements will be performed, see p 260-289 in the lab text for more information on IR.<br />
H3C<br />
t-Amyl Chloride: Page 1<br />
CH3<br />
CH2<br />
Cl<br />
CH 3<br />
+ H2O
Helpful Hints - t-Amyl chloride<br />
* Whenever a separatory funnel is used, do not discard any fraction unless you can account for your<br />
product. An easy method to ascertain which layer is which is the following: Add about 2-3 mL of water<br />
into a test tube, and place the test tube lip around the "takeout arm" of your separatory funnel. The<br />
bottom layer is released through the stopcock into the test tube containing the water. Upon agitation,<br />
observe whether the mixture is homogeneous. By deductive reasoning, you now know (hopefully)<br />
which layer is which!!<br />
SYN<strong>THE</strong>SIS OF tert-<strong>AMYL</strong> <strong>CHLORIDE</strong> - HOMEWORK QUESTIONS<br />
Tertiary alkyl bromides can be prepared from the corresponding alcohol by the reaction with concentrated<br />
hydrobromic acid instead of conc.. hydrochloric acid as shown by the following equation.<br />
(1) Consider the above reaction using 25.0 mL of tert-butyl alcohol (d = 0.786 g/mL) with 60.0 mL of<br />
concentrated hydrobromic acid (d = 1.49 g/mL, 47.0% HBr). On a separate sheet calculate the theoretical<br />
yield in grams and the percent yield for a reaction that produced 26.1 g of tert-butyl bromide. Clearly show<br />
the set ups to determine the limiting reactant and other calculations using proper units and significant<br />
figures.<br />
Consult your textbook for the following TWO synthesis. Keep in mind that conc. HCl and conc. HBr<br />
will NOT give good yields of alkyl halides by the reaction with primary and secondary alcohols.<br />
(2) Give the balanced equation to prepare chlorocyclohexane in good yield from cyclohexanol.<br />
(3) Give the balanced equation for the preparation in good yield of 1-bromopentane from 1-pentanol.<br />
(4) The reverse of the reaction you performed in the lab can also occur. Under the proper conditions,<br />
tertiary alkyl halides may undergo a hydrolysis reaction to form an alcohol and a hydrogen halide.<br />
Complete and balance the following equation. (Remember the General II experiment?)<br />
t-Amyl Chloride: Page 2