chemical physics of discharges - Argonne National Laboratory
chemical physics of discharges - Argonne National Laboratory chemical physics of discharges - Argonne National Laboratory
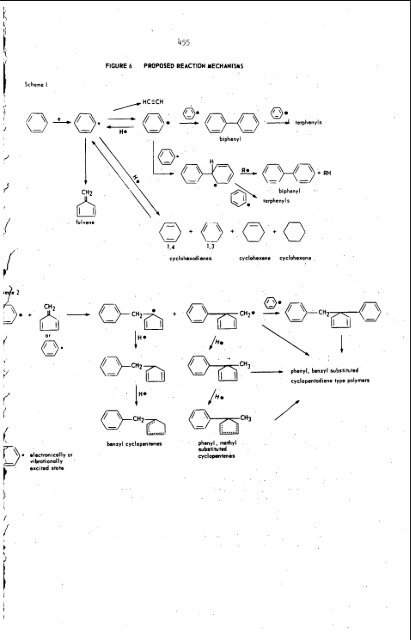
’. 454 Mechanism I The available evidence is thus not consistent with the generally proposed i-iiechaii;si;i 0: i;~:j.~~i f~iii~atisii based ~ ithe i iaiidoiii bui:d-i;p d 2 PO:? (pheiijjl~iiej chain 1 accompanied by hydrogenation, or the interaction with the hexatrienyl diradi~al.~’ In the radiofrequency discharge of benzene, evidence was presented which clearly indicated that the polymer contained consecutive para linkages suggesting poly (p-phepylenes ) as the predominant structure.12 Schcler observed that the polymer (C H 1.03, M.W. 503) was a phenyl substituted aliphatic chain based on infrared evidence.16 Patrick and Burton have ‘ demonstrated that hydrogen atoms are not involved to any significant extent in polymer formation when liquid benzene is irradiated with a 1.5 m.e.v. source. 24 \ In the corona discharge many types of energy transfer are occurring, varying from photolysis (visible corona) to relatively high energy electrons responsible for frag- ‘i mentation. The low yield of biphenyl in this single pass reactor suggests that phenyl radical ’ production through loss of a hydrogen radical is not the primary reaction route leading to polymer formation. Considerable energy should be available to excite benzene to relatively high vibrational levels which would be somewhat below that energy required to actually -\ separate. the H radical. At any given time the number of these excited benzene molecules should greatly exceed the phenyl radical population. Fulvene is produced from benzene by ultra-violet energy (200 mp, about 112 kcals, or 4.9 electron volts).25 Thus, the formation of this benzene isomer with a resonance energy (11-12 kcals/mole) intermediate between benzene and 1,3 cyclohexadiene may be energetically favorable in the corona environment. \\ 1 Fulvene has been shown to polymerize rapidly under similar conditions with no reversion to benzene.25 The uniformity of the phenyl (benzyl) -cyclopentene ratio throughout all polymer fractions makes it difficult to accept a mechanism based solely on random attack 1 by phenyl radicals on the growing fulvene polymer. The initial synthesis of a monomeric unit comprised of the benzylcyclopentadiene system with subsequent diene type polymerization is consistent with all our observations. The phenyl radical produced in the discharge should collide with the nearest benzene molecule which will be excited to a \ relatively high vibrational energy level. The following reaction sequences are suggested for the phenyl radical: U Re*=+ Reaction (5) is the typical termination reaction by radical coupling leading to biphenyl. The intermediate radical in (6) would be expected to lose hydrogen mo e readily than it would add to another benzene molecule to give polymer formation.2d The possibility of ring contraction in the excited intermediate, accompanied by an intramolecular shift of a hydrogen atom is suggested in reaction (7). The benzylcyclopentadiene XV so obtained would be a very active monomer leading to the polymeric products observed. Although our data support polymer formation by the mechanism indicated, we cannot rule out participation by the cyclohexadienes and acetylene, both of these being noted a - e n e - CkEketewr-eentper-at-. XIV
1' b Scheme I fulvene 455 FIGURE 6 PROPOSED REACTION MECHANISMS I iHO 1,4 1.3 biphenyl A\ biphenyl '. 0.- terphenyls cyclohoxodienes cycloherene cyclohexane @z, /I P ) 7 3 C H 3 . . .. . . .. . benzyl cyclopentenes / \ 05CH3- phenyl, methyl substituted cyclapentenis phenyl, benzylrubstlhlted u cyclopentadiene type polymers /
- Page 408 and 409: 404 Before startup, the system is p
- Page 410 and 411: 4 OL. ' , I. . TesCs. With.Coals .o
- Page 412 and 413: Table 3.- Product Gas Analyses and
- Page 414 and 415: 410 BIBLIOGRAPHY 1. American Public
- Page 416 and 417: Figure 2. Enclosure surrounding hyd
- Page 418 and 419: 0 0 f v) C 0 u m I z 2 c 2 r d J w
- Page 420 and 421: 0. E c .4 7 .. .c . I ( - Low tempe
- Page 422 and 423: 418 cual. A mjor aa\;antage or' the
- Page 424 and 425: 420 Ir ?Y ? ? 9 9 9 pc rod n o c o
- Page 426 and 427: 422 Table 3. Room-texperature M'dss
- Page 428 and 429: 424 state, depending on the strengt
- Page 430 and 431: Table 4. Room-?;ergerature isomer s
- Page 432 and 433: I' 1.3 . /o 9 IO 9 6 a 425 F F 33 I
- Page 434 and 435: Brooks J.D. and Sternhell S. (1957)
- Page 436 and 437: (52) Gib3 T.C. and Greenwood N.N. (
- Page 438 and 439: 434 transducer vas then introduced
- Page 440 and 441: 436 yield is quite similar. The con
- Page 442 and 443: ?-!??? 0 mv, Uh\OhIe . . . . eirlrl
- Page 444 and 445: 4. 5. 6. 7. 8. 9. 10. 11. 12. 13. 4
- Page 446 and 447: 442 CHEMICAL REACTIONS IN A CORONA
- Page 448 and 449: helium 444 CORONA REACTOR SYSTEM Fi
- Page 450 and 451: 446 The ultraviolet spectrum. for t
- Page 452 and 453: Biphenyl Fraction 448 The low molec
- Page 454 and 455: LUd The actual structural definitio
- Page 456 and 457: I I I I I In m 9 In 2 , I: r- In m
- Page 460 and 461: 456 , , . The relatively low yield
- Page 462 and 463: 458 h/lethylacetylene, allene and b
- Page 464 and 465: Introduction VAPOR PHASE DECOMPOSIT
- Page 466 and 467: 462 more efficient hydrogenation. T
- Page 468 and 469: 464 place in parallel with fragment
- Page 470 and 471: 466 P rl 0, k 7 rnI rn al k a I hl
- Page 472 and 473: 458 TABLE I. COMPOSI'IION OF GASEOU
- Page 474 and 475: REFERENCES 1. C. Hirayama and D. A.
- Page 476 and 477: i 472 Reciprocal Arc Enthalpy ,-> F
- Page 478 and 479: 474 be pumped down without altering
- Page 480 and 481: 476 Instead a hydrogen deficient fi
- Page 482 and 483: i 478
- Page 484 and 485: 480 FATTY ACIDS AND n-ALKANES IN GR
- Page 486 and 487: 482 TA5L,E 2. - Carbon number distr
- Page 488 and 489: 6. 7. a. 9. 484 Lawlor, D. L., and
- Page 490 and 491: ' I ' Sample No. 6 ' 12 IO 8 6 z 4
- Page 492 and 493: . 488 uiolecular weight of which ca
- Page 494 and 495: Xississippi (Sample GS). Stock solu
- Page 496 and 497: 492 found at 5.7 and 4.9& for the W
- Page 498 and 499: 494 mechanisms. However, the voltad
- Page 500 and 501: For pure polynuclcar arom.i;ic hydr
- Page 502 and 503: - '-'. . ' . . values are listed in
- Page 504 and 505: 500 (1;) Ten, T. 17.: a;1d,Dic;
- Page 506 and 507: - ~ Resistivity Czlculation 2: 1 j:
1'<br />
b<br />
Scheme I<br />
fulvene<br />
455<br />
FIGURE 6 PROPOSED REACTION MECHANISMS<br />
I<br />
iHO<br />
1,4 1.3<br />
biphenyl<br />
A\ biphenyl '. 0.- terphenyls<br />
cyclohoxodienes cycloherene cyclohexane<br />
@z, /I P ) 7 3 C H 3<br />
. . .. . . .. . benzyl cyclopentenes<br />
/ \ 05CH3-<br />
phenyl, methyl<br />
substituted<br />
cyclapentenis<br />
phenyl, benzylrubstlhlted<br />
u cyclopentadiene type polymers<br />
/