chemical physics of discharges - Argonne National Laboratory
chemical physics of discharges - Argonne National Laboratory chemical physics of discharges - Argonne National Laboratory
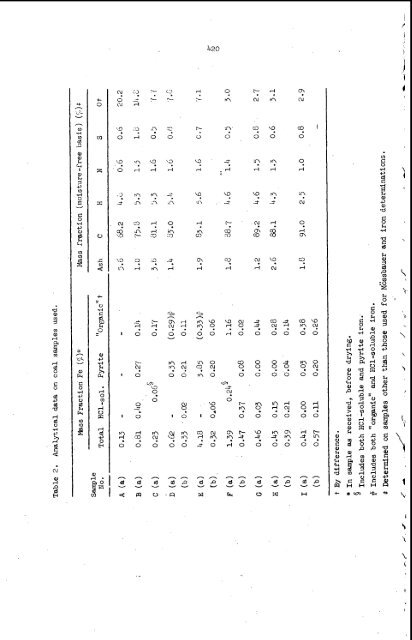
420 Ir ?Y ? ? 9 9 9 pc rod n o c o o 0 Y 0 00 M O . o 0 -f. H ' O 9 0 I O I O 0 0 0 0;' N c9 0 9 rl ;? N 9 rl m 0. rl f co'O ? E?? 0 0 0 f n o 9 ?Y 0 0 0 O F lnd O d '?? ?cu. ? ? 0 0 0 0 0 0 I
L. 421 minutes, yielding c 600,000 counts and a relative standard deviation < 0.135. The velocity region scanned for each sauple was from -2.OC mm s-l to about + 4.00 mm s-'. Most measurements were made a rooi; temperature. A cryostat mde from I' S t y r O f a $as mounted on the moveable table 0: the instrument ;or low-terqerature I measurements. The sample was placed in this and submerged in liquid N2. The , spectrum of each coal sample was run twice at room temperature, befcke and after , the spectm was taken at liquid42 temperature. Spectral parameters were generally read from plotted spectra. The data from sample F(a) were fitted to a double Lorentz curve by a least-squares program using J an IB1 7C40 computer. Isomer shifts are reported (in m s-') with respect to the , ismer shift of sodium nitroprusside. Quadrupole splittings are reported (in mm s-') as the velocity differences between the minima of two associated absorption lines. I DATA / Room-temperature spectra for the nine coal samples are illustrated in Figure 1. J Each spectrum shows neither, either, or both of just two components: (1) a close doublet similar to those of pyrite and mrcasite, and (2) a wide doublet similar to those of many ferrous compounds. Table 3 lists the Mossbauer paramters, including the fractional peak absorptions, for what we will call respectively the pyrite and non-pyrite resonances. Isomer shifts and quadrupole splittings obtained / with our instrument on sane powdered iron campounds and minerals which were regarded as possibilities for the non-pyrite spectrum appear in Table 4. J / The parameters observed for the pyrite iron are: isomer shift (d) = +0.54 mm s-l; quadrupole splitting (A) = 0.58 m s-I. All of the coals having a non- ' pyrite absorption as shown in Figure 1 have: d = +l.38 mm s-'; A = 2.62 mm s-'. p' The computer analysis of sample F(a) indicated both lines in the spectrum had c widths (r) of 0.39 mm s-l; their intensity ratio is within 57; of unity. I i Liquid-N2 spectra showed the same absorption peaks as at room temperature, f with d = +0.65 m s-l and A = 0.58 mm s-l for pyrite and d = +1.47 mm s-' and > A = 2.78 mm s-l for the non-pyrite iron. INTERPRETATION i Y The isomer shift is a function of nuclear properties and the electron density at the absorbing nucleus relative to that of the source or a standard absorber, such as sodium nitroprusside (23,24). As the electron density at the iron nucleus, due essentially only to s electrons, increases, the isomer shift decreases '[algebraically. Thus, ferrous compounds have a more positive isomer shift than ferric compounds, as the additional 3d electron of the former increases the d shielding effect on the 3s electrons and thereby decreases their density at the f nucleus (18). For ferric iron, isomer shifts (relative to sodium nitroprusside) \ have been observed in the range 4.1 to +1.1 mm S'l, and for ferrous iron from -0.1 to +1.6 mm S-'. i; I Quadrupole splitting into a two-line spectrum occurs when the iron nucleus he field consists of two parts, (1) that f is in an asymmetric electric field. i produced by the electrons of the iron atom, including those shared with adjacent 1 atoms, and (2) that resulting from charges of the surrounding atoms; each part J can contribute to asymmetry at the nucleus. Ferric compounds shm quadrupole splittings from zero to about 2.3 nun s-l; here the asymmetry is caused chiefly by charges of the surrounding atoms. Ferrous compounds show larger values, frm zero Ferrous iron can exist in either a high-spin or a low-spin J 1 i I r I to - 3.3 mm s-I.
- Page 374 and 375: . . I . I 370 . . . . ., . . .. . .
- Page 376 and 377: 4 372 090- > E w - m c N O - 0 z :
- Page 378 and 379: A-Feed tank 6-Thermocracker GReceiv
- Page 380 and 381: 80 \ 0 70 60 e 40 a U VI 9 30 20 10
- Page 382 and 383: COAL DEASH& AND HIGH-PURITY COKE Wa
- Page 384 and 385: 3Pn _. -. - L -J:,J.-;~~~L, ):as se
- Page 386 and 387: S ~ l ~ o n~pg:ading r is the resul
- Page 388 and 389: The range ~f temperatures and gener
- Page 390 and 391: . .. 386 9 * - r. Y 2 .' d a f' r.
- Page 392 and 393: .) .L m 388 I
- Page 394 and 395: TABLE 4 390 DELAYED COKING OF DEASH
- Page 396 and 397: , ! I- on '---
- Page 398 and 399: 394 The present method is to keep t
- Page 400 and 401: 396 ‘c erperature, while the pres
- Page 402 and 403: 2, '! ! Proximate Analysis, wt $ Mo
- Page 404 and 405: 0 IS 0 14 LL 9 013 e \ 3 b- m *- 01
- Page 406 and 407: 402 HYDROGEN CYANIDE PRODUCED FROM
- Page 408 and 409: 404 Before startup, the system is p
- Page 410 and 411: 4 OL. ' , I. . TesCs. With.Coals .o
- Page 412 and 413: Table 3.- Product Gas Analyses and
- Page 414 and 415: 410 BIBLIOGRAPHY 1. American Public
- Page 416 and 417: Figure 2. Enclosure surrounding hyd
- Page 418 and 419: 0 0 f v) C 0 u m I z 2 c 2 r d J w
- Page 420 and 421: 0. E c .4 7 .. .c . I ( - Low tempe
- Page 422 and 423: 418 cual. A mjor aa\;antage or' the
- Page 426 and 427: 422 Table 3. Room-texperature M'dss
- Page 428 and 429: 424 state, depending on the strengt
- Page 430 and 431: Table 4. Room-?;ergerature isomer s
- Page 432 and 433: I' 1.3 . /o 9 IO 9 6 a 425 F F 33 I
- Page 434 and 435: Brooks J.D. and Sternhell S. (1957)
- Page 436 and 437: (52) Gib3 T.C. and Greenwood N.N. (
- Page 438 and 439: 434 transducer vas then introduced
- Page 440 and 441: 436 yield is quite similar. The con
- Page 442 and 443: ?-!??? 0 mv, Uh\OhIe . . . . eirlrl
- Page 444 and 445: 4. 5. 6. 7. 8. 9. 10. 11. 12. 13. 4
- Page 446 and 447: 442 CHEMICAL REACTIONS IN A CORONA
- Page 448 and 449: helium 444 CORONA REACTOR SYSTEM Fi
- Page 450 and 451: 446 The ultraviolet spectrum. for t
- Page 452 and 453: Biphenyl Fraction 448 The low molec
- Page 454 and 455: LUd The actual structural definitio
- Page 456 and 457: I I I I I In m 9 In 2 , I: r- In m
- Page 458 and 459: ’. 454 Mechanism I The available
- Page 460 and 461: 456 , , . The relatively low yield
- Page 462 and 463: 458 h/lethylacetylene, allene and b
- Page 464 and 465: Introduction VAPOR PHASE DECOMPOSIT
- Page 466 and 467: 462 more efficient hydrogenation. T
- Page 468 and 469: 464 place in parallel with fragment
- Page 470 and 471: 466 P rl 0, k 7 rnI rn al k a I hl
- Page 472 and 473: 458 TABLE I. COMPOSI'IION OF GASEOU
420 Ir<br />
?Y ? ? 9 9 9<br />
pc rod n o c o o 0<br />
Y<br />
0 00 M O<br />
. o<br />
0<br />
-f.<br />
H ' O 9<br />
0 I O I O<br />
0 0 0<br />
0;'<br />
N<br />
c9<br />
0<br />
9<br />
rl<br />
;?<br />
N<br />
9<br />
rl<br />
m<br />
0.<br />
rl<br />
f co'O<br />
? E??<br />
0 0 0<br />
f n o<br />
9 ?Y<br />
0 0 0<br />
O F lnd O d<br />
'?? ?cu. ? ?<br />
0 0 0 0 0 0<br />
I