chemical physics of discharges - Argonne National Laboratory
chemical physics of discharges - Argonne National Laboratory chemical physics of discharges - Argonne National Laboratory
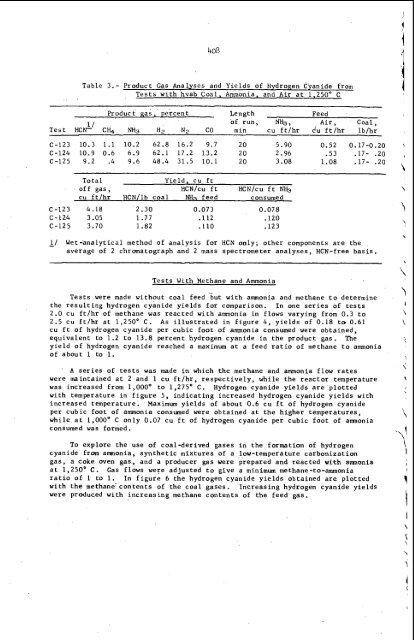
Table 3.- Product Gas Analyses and Yields of Hydrogen Cyanide from Tests with hvab Coal. Ammonia, and Air at 1,250" C Product gas, percent Length Feed of run, N&, Air, Coal, Test HC&' C& NH3 H2 N2 CO min cu ft/hr du ft/hr lb/hr C-123 10.3 1.1 10.2 62.8 16.2 9.7 20 5.90 0.52 0.17-0.20 C-124 10.9 0.6 6.9 62.1 17.2 13.2 20 2.96 .53 .17- .20 C-125 9.2 .4 9.6 48.4 31.5 10.1 20 3.08 1.08 .17- .20 Total Yield, cu ft . off gas, HCN/cu ft HCN/cu ft N& cu ft/hr HCN/lb coal NKq feed c on s ume d C-123 4.18 2.30 0.073 0.078 C-124 3.05 1.77 .112 .120 C-125 3.70 1.82 .110 .123 - 1/ Wet-analytical method of analysis for HCN only; other components are the average of 2 chromatograph and 2 mass spectrometer analyses, HCN-free basis. Tests With Methane and Ammonia Tests were made without coal feed %ut with amnonia and methane to determine the resulting hydrogen cyanide yields for comparison. In one series of tests 2.0 cu ft/hr of methane was reacted with ammonia in flows varying from 0.3 to 2.5 cu ft/hr at 1,250' C. As illustrated in figure 4, yields of 0.18 to 0.61 cu ft of hydrogen cyanide per cubic foot of ammonia consumed were obtained, equivalent to 1.2 to 13.8 percent hydrogen cyanide in the product gas. The yield of hydrogen cyanide reached a maximum at a feed ratio of methane to amonia of about 1 to 1. ' A series of tests was made in which the methane and ammonia flow rates were maintained at 2 and 1 cu ft/hr, respectively, while the reactor temperature was increased from l,OOOo to 1,275' C. Hydrogen cyanide yields are plotted with temperature in figure 5, indicating increased hydrogen cyanide yields with increased temperature. Maximum yields of about 0.6 cu ft of hydrogen cyanide per cubic foot of ammonia consumed were obtained at the higher temperatures, while at 1,000" C only 0.07 cu ft of hydrogen cyanide per cubic foot of amnonia consumed was formed. To explore the use of coal-derived gases in the formation of hydrogen cyanide from amnonia, synthetic mixtures of a low-temperature carbonization gas, a coke oven gas, and a producer gas were prepared and reacted with amnonia at 1.250" C. Gas flows were adjusted to give a minimum methane-to-ammonia ratio of 1 to 1. In figure 6 the hydrogen cyanide yields obtained are plotted with the methane' contents of the coal gases. Increasing hydrogen cyanide yields were produced with increasing methane contents of the feed gas. \ \
409 ECONOMIC EVALUATION The Bureau of Mines Process Evaluation Group: Morgantown, W. Va., made a preliminary cost study of an integrated plant to produce hydrogen cyanide by reaction of ammonia with coal. The cost study was based on experimental results including a yield of 0.6 cu ft of hydrogen cyanide per cubic foot of ammonia. Electrical heating was assumed as in the becch-scale tests; a plant capacity of 40 million pounds per year was chosen. The total estimated capital investment was $12,930,000 including costs for power generation. Based on a coal cost of $4.00 per ton and an ammonia cost of $100.00 per ton, the operating costs before profit and taxes would be 5.82 cents per pound of hydrogen cyanide product allowing byproduct credit. Addition of 12-percent gross return on investment would give production costs of 9.7 cents per pound of product when $4.00 per ton coal is used. The current market price is 11.5 cents per pound.81 Credit has been allowed in the cost figures for a 7.6-percent yield of carbon black and the excess char produced in the process. Some of the char and the scrubbed product gas (containing about 75 percent hydrogen) are consumed in the steam plant for power generation. Electrical heating, which was used in the test unit and also in the cost figures, is one of the most expensive types of heating, accounting for greater than 40 percent of the capital costs in the estimate. If cheaper conventional heating could be used, production costs would be lowered considerably. CONCLUSIONS Hydrogen cyanide has been produced from coal and amnonia at 1,250" C in bench-scale studies. The use of a metal reactor was unsuccessful because the metal failed at the temperatures required, and the yield of hydrogen cyanide was low. The yield was improved greatly when a refractory ceramic reactor was used. Hydrogen cyanide yields approximating stoichiometric of 1 cu ft of hydrogen cyanide per cubic foot of ammonia reacted were obtained at low flows of ammonia. At higher amnonia flows, ammonia conversion of about 75 percent was obtained, which is the usual conversion attained in commercial units using natural gas and a platinum catalyst. The low-volatile coals gave low yields of hydrogen cyanide; the high- volatile coals gave the best yields. The results indicated that the hydrogen cyahi.de is produced by reaction of amnonia with the hydrocarbons in the coal. Yields of hydrogen cyanide from reaction of ammonia with gas mixtures con- taining methane are directly related to the methane content of the gas. Cost studies indicate that hydrogen cyanide can be produced from coal and aunnonia at a price approximating the posted sales price.
- Page 362 and 363: I 1 \ 361 -. ne reaction proceecis
- Page 364 and 365: \ 36 3 ' h, I 26. H. Purnell, Gas C
- Page 366 and 367: In experiments on the direct conver
- Page 368 and 369: 367 used as blnders, with different
- Page 370 and 371: 369 ' i 1. REFERENCES Berber, John
- Page 372 and 373: Distribution and yield rates of pro
- Page 374 and 375: . . I . I 370 . . . . ., . . .. . .
- Page 376 and 377: 4 372 090- > E w - m c N O - 0 z :
- Page 378 and 379: A-Feed tank 6-Thermocracker GReceiv
- Page 380 and 381: 80 \ 0 70 60 e 40 a U VI 9 30 20 10
- Page 382 and 383: COAL DEASH& AND HIGH-PURITY COKE Wa
- Page 384 and 385: 3Pn _. -. - L -J:,J.-;~~~L, ):as se
- Page 386 and 387: S ~ l ~ o n~pg:ading r is the resul
- Page 388 and 389: The range ~f temperatures and gener
- Page 390 and 391: . .. 386 9 * - r. Y 2 .' d a f' r.
- Page 392 and 393: .) .L m 388 I
- Page 394 and 395: TABLE 4 390 DELAYED COKING OF DEASH
- Page 396 and 397: , ! I- on '---
- Page 398 and 399: 394 The present method is to keep t
- Page 400 and 401: 396 ‘c erperature, while the pres
- Page 402 and 403: 2, '! ! Proximate Analysis, wt $ Mo
- Page 404 and 405: 0 IS 0 14 LL 9 013 e \ 3 b- m *- 01
- Page 406 and 407: 402 HYDROGEN CYANIDE PRODUCED FROM
- Page 408 and 409: 404 Before startup, the system is p
- Page 410 and 411: 4 OL. ' , I. . TesCs. With.Coals .o
- Page 414 and 415: 410 BIBLIOGRAPHY 1. American Public
- Page 416 and 417: Figure 2. Enclosure surrounding hyd
- Page 418 and 419: 0 0 f v) C 0 u m I z 2 c 2 r d J w
- Page 420 and 421: 0. E c .4 7 .. .c . I ( - Low tempe
- Page 422 and 423: 418 cual. A mjor aa\;antage or' the
- Page 424 and 425: 420 Ir ?Y ? ? 9 9 9 pc rod n o c o
- Page 426 and 427: 422 Table 3. Room-texperature M'dss
- Page 428 and 429: 424 state, depending on the strengt
- Page 430 and 431: Table 4. Room-?;ergerature isomer s
- Page 432 and 433: I' 1.3 . /o 9 IO 9 6 a 425 F F 33 I
- Page 434 and 435: Brooks J.D. and Sternhell S. (1957)
- Page 436 and 437: (52) Gib3 T.C. and Greenwood N.N. (
- Page 438 and 439: 434 transducer vas then introduced
- Page 440 and 441: 436 yield is quite similar. The con
- Page 442 and 443: ?-!??? 0 mv, Uh\OhIe . . . . eirlrl
- Page 444 and 445: 4. 5. 6. 7. 8. 9. 10. 11. 12. 13. 4
- Page 446 and 447: 442 CHEMICAL REACTIONS IN A CORONA
- Page 448 and 449: helium 444 CORONA REACTOR SYSTEM Fi
- Page 450 and 451: 446 The ultraviolet spectrum. for t
- Page 452 and 453: Biphenyl Fraction 448 The low molec
- Page 454 and 455: LUd The actual structural definitio
- Page 456 and 457: I I I I I In m 9 In 2 , I: r- In m
- Page 458 and 459: ’. 454 Mechanism I The available
- Page 460 and 461: 456 , , . The relatively low yield
Table 3.- Product Gas Analyses and Yields <strong>of</strong> Hydrogen Cyanide from<br />
Tests with hvab Coal. Ammonia, and Air at 1,250" C<br />
Product gas, percent Length Feed<br />
<strong>of</strong> run, N&, Air, Coal,<br />
Test HC&' C& NH3 H2 N2 CO min cu ft/hr du ft/hr lb/hr<br />
C-123 10.3 1.1 10.2 62.8 16.2 9.7 20 5.90 0.52 0.17-0.20<br />
C-124 10.9 0.6 6.9 62.1 17.2 13.2 20 2.96 .53 .17- .20<br />
C-125 9.2 .4 9.6 48.4 31.5 10.1 20 3.08 1.08 .17- .20<br />
Total Yield, cu ft<br />
. <strong>of</strong>f gas, HCN/cu ft HCN/cu ft N&<br />
cu ft/hr HCN/lb coal NKq feed c on s ume d<br />
C-123 4.18 2.30 0.073 0.078<br />
C-124 3.05 1.77 .112 .120<br />
C-125 3.70 1.82 .110 .123<br />
- 1/ Wet-analytical method <strong>of</strong> analysis for HCN only; other components are the<br />
average <strong>of</strong> 2 chromatograph and 2 mass spectrometer analyses, HCN-free basis.<br />
Tests With Methane and Ammonia<br />
Tests were made without coal feed %ut with amnonia and methane to determine<br />
the resulting hydrogen cyanide yields for comparison. In one series <strong>of</strong> tests<br />
2.0 cu ft/hr <strong>of</strong> methane was reacted with ammonia in flows varying from 0.3 to<br />
2.5 cu ft/hr at 1,250' C. As illustrated in figure 4, yields <strong>of</strong> 0.18 to 0.61<br />
cu ft <strong>of</strong> hydrogen cyanide per cubic foot <strong>of</strong> ammonia consumed were obtained,<br />
equivalent to 1.2 to 13.8 percent hydrogen cyanide in the product gas. The<br />
yield <strong>of</strong> hydrogen cyanide reached a maximum at a feed ratio <strong>of</strong> methane to amonia<br />
<strong>of</strong> about 1 to 1.<br />
' A series <strong>of</strong> tests was made in which the methane and ammonia flow rates<br />
were maintained at 2 and 1 cu ft/hr, respectively, while the reactor temperature<br />
was increased from l,OOOo to 1,275' C. Hydrogen cyanide yields are plotted<br />
with temperature in figure 5, indicating increased hydrogen cyanide yields with<br />
increased temperature. Maximum yields <strong>of</strong> about 0.6 cu ft <strong>of</strong> hydrogen cyanide<br />
per cubic foot <strong>of</strong> ammonia consumed were obtained at the higher temperatures,<br />
while at 1,000" C only 0.07 cu ft <strong>of</strong> hydrogen cyanide per cubic foot <strong>of</strong> amnonia<br />
consumed was formed.<br />
To explore the use <strong>of</strong> coal-derived gases in the formation <strong>of</strong> hydrogen<br />
cyanide from amnonia, synthetic mixtures <strong>of</strong> a low-temperature carbonization<br />
gas, a coke oven gas, and a producer gas were prepared and reacted with amnonia<br />
at 1.250" C. Gas flows were adjusted to give a minimum methane-to-ammonia<br />
ratio <strong>of</strong> 1 to 1. In figure 6 the hydrogen cyanide yields obtained are plotted<br />
with the methane' contents <strong>of</strong> the coal gases. Increasing hydrogen cyanide yields<br />
were produced with increasing methane contents <strong>of</strong> the feed gas.<br />
\<br />
\