chemical physics of discharges - Argonne National Laboratory
chemical physics of discharges - Argonne National Laboratory chemical physics of discharges - Argonne National Laboratory
323 ....... - . . . . . . . . . . . . . . . ........... i . . . . . . . . . . . . . . . . . . ................ -., ......................
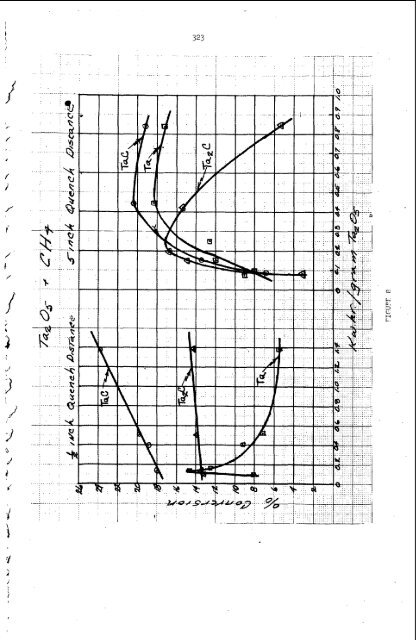
324 5 Toes u? linearallv with the Kihr/p input. Where the Quenching distance was 5" a Deak was obtained which shows that adequate quenchinrr does not take place bevond a value of 0.4 for Kwhr/p. Note that in the second case the formation of Ta2C also peaks and then Falls off rapidly, in contrast to the 1/2" distance. Tantalum metal is the favored product in the 5" case. and 18% %a for both cases. Maximum conversions are 24% TaC, 17% Ta2C The reduction of tantalum pentoxide with hvdroEen (12) in 7 helium plasma jet to produce tantalum metal (reaction A) is shown in Pipure 9. P.@n the ? conversion is plotted vs. Kwhr/p Ta2O5 input for two different quenching distances. As can be seen the more rapid quenching (1/2". case) gives the maximum conversion (42%). which peaks at 0.35 Kwhr/g Tap05. In a similar manner the reduction of tunpsten trioxide was carried out in a ) '1 \ ' helium plasma jet (17) with the quenching device 5" below the plasma jet. Conversions as hiKh as 959, were obtained carryinp the tunesten trioxide in hydroaen into the ( flame of the 'et. The reduction of other metal oxides has also bean experimentally investigated 117). Ferric oxide was reduced to iron metal in a 100% conversion '\ using a helium plasma jet and carrvinp the ferric oxide in hvdroEen (reaction 11). Titanium dioxide and zirconium dioxide reductions were also attempted hv the same method, however, no reduction was obtained in either case. The reduction of aluminum i , oxide with hydropen in a helium plasma jet produced only a 2 to 5% conversion to aluminum metal usinp several different quenching methods. Pydrocarbons C2H2 LIOUIDIGAS FEACTIONS PRODUCING A GAS The aroduction of ozone b means of a plasma jet was accomplished by feeding, liquid oxyEen into a helium jet (1* v , Figure 10 shows the schematic of the apparatus used and Fipure 11 shows the effect of liquid oxypen flow on ozone production under constant arc conditions. The liquid oxygen acts as both a reactant and quenching mediu Another example of this type reactant is the decomposition of hydrocarbons into acetylene by use of a plasma jet device. Thermodynamics Corporation (19) has proved \ the feasibility of producinp acetylene from kerosene using a plasma torch. Preliminary runs gave yields of 18% acetylene. II II LIOUID-GAS REACTIONS TO PRODUCE A LIQUID \ 1
- Page 273 and 274: 273 ion (1L.). Zxcitei-ion by colli
- Page 275 and 276: Apparatus and Procedure 275 EXPERIM
- Page 277 and 278: . 277 6. A freshly prepared sam le
- Page 279 and 280: observed. The polystyrene (10% solu
- Page 281 and 282: i i (12) c (11) (14) I 281 LITERATU
- Page 283 and 284: FLOW- METER I ADDITIVE SUPPLY * ,28
- Page 285 and 286: 285 TABLE 2 Effect of Haloqenated A
- Page 287 and 288: ANALYTICAL RESULTS Some evidence wa
- Page 289 and 290: DISCUSSION 289 The salient features
- Page 291 and 292: ? ~ MENBERSHIP IN THE DIVISION OF F
- Page 293 and 294: 292 flow of the reactant gas stream
- Page 295 and 296: 294 have suitable residence times i
- Page 297 and 298: 0 E < h( E l! d K c 0 .- Y 0 t u t
- Page 299 and 300: 298 yields of many chemical product
- Page 301 and 302: - ;i Y . 15. Pressure lOmm 5 > 10-
- Page 303 and 304: 302 the best and in many practical
- Page 305 and 306: GENERATION AND MEASUREMENT OF AUDIO
- Page 307 and 308: . The transformer secondary and the
- Page 309 and 310: Vaccum-Tube Amplifier The mobile co
- Page 311 and 312: 310 f = Power supply frequency in c
- Page 313: FUFARCI! INSTITUTE OF TFYDLC UNIVER
- Page 316 and 317: J # I / 4) I . . '8 r4 0 r( P
- Page 318 and 319: 317 i Cuencb svsten w.nnar;itus use
- Page 320 and 321: epcrte? the noss~kil.itv of nroduct
- Page 322 and 323: 1' b t +
- Page 326 and 327: 1 \ i I i , / / / 8 3 1 325 To205 +
- Page 328 and 329: - .- - - . - - . . . - . 327 n + c
- Page 330 and 331: ' POWER PLASMA GAS l e , ' I ! I I
- Page 332 and 333: 331 f'ENI?AL PF'FCLCCFC Dernis, P.R
- Page 334 and 335: i J 333 ; mosphere (as opposed to a
- Page 336 and 337: ; it raised the question, still &?z
- Page 338 and 339: p .I V I .= - - HCN/C(CALC. WITH C
- Page 340 and 341: 1 1 339 atmospheric ressure and ach
- Page 342 and 343: RESULTS 341 Composition Dependence
- Page 344 and 345: 0.09 0.08 0.07 9 0.06 2 U - .$ 0.05
- Page 346 and 347: II \ to the Slot So that less of th
- Page 348 and 349: i 4 2 HYDROCARBON-NITROGEN REACTION
- Page 350 and 351: I 1 349 c M m ; a e, k M x
- Page 352 and 353: 1.0 I - a. I n TEMPERATURE - OK Fig
- Page 354 and 355: 353 the experimental results in the
- Page 356 and 357: ,) 355 The ceaswed conversion cf ni
- Page 358 and 359: 1 6 357 Freezing. 3jpotnesise thzf
- Page 360 and 361: 359 \ Frozen Compositior: Calcillat
- Page 362 and 363: I 1 \ 361 -. ne reaction proceecis
- Page 364 and 365: \ 36 3 ' h, I 26. H. Purnell, Gas C
- Page 366 and 367: In experiments on the direct conver
- Page 368 and 369: 367 used as blnders, with different
- Page 370 and 371: 369 ' i 1. REFERENCES Berber, John
- Page 372 and 373: Distribution and yield rates of pro
323<br />
....... -<br />
. . . . . . . . . . . . . . . ...........<br />
i<br />
. .<br />
. . .<br />
. . . . . . . . . . . . . ................ -., ......................